The discharge permission concentration for nitrogen and phosphorus becomes strict gradually in wastewater. Many conventional wastewater treatment technologies have been developed to meet the stringent discharge permit limitation [1]. In wastewater treatment technology, various techniques have been used for nitrogen and phosphorus removal. Among these, chemical and biological methods have been successfully applied [2]. However, many existing removal processes of nitrogen and phosphorus were progressed to multi-stage system because of difficulty in simultaneous removal of nitrogen and phosphorus. To solve such a defect, we investigated the simultaneous removal of ammonium, nitrate and phosphorus through one-stage system by using the surface modified zeocarbon.
The zeocarbon is mixture of zeolite and activated carbon.Expatiate on, it is made in spherical shape granule after zeolite and activated carbon were mixed by inorganic binder. Accordingly, the zeocarbon has superior adsorption capacity and excellent cation exchange capacity due to activated carbon and zeolite. At present, the zeocarbon is using for removal of low concentration of ammonia, amine, carbon monoxide and nitric oxide compound by adsorption. Also, the zeocarbon is applying for maintenance of the freshness and is applying for cigarette filter [3]. However, the commercial zeocarbon has limitations in applying for water treatment because of absorbent itself was produced for gas phase adsorption. The limitation of zeocarbon is as following concretely. Firstly, strength of zeocarbon become less in the presence of water for a long time and incipient spherical shape of zeocarbon is not maintaining. These indicate that recovery and regeneration of the zeocarbon is difficult. The second, high pH value and turbidity of effluent water by liquation of a component of the zeocarbon leads to difficulty in application for practical process. Thereupon, the surface of zeocarbon was modified by acid to apply for water treatment. The surface modified zeocarbon was used for simultaneous removal of ammonium, nitrate and phosphorus in water. One of the main advantages of ammonium, nitrate and phosphorus removal by using the zeocarbon over the other chemical treatment methods is that it does not produce any excess chemical sludge as precipitation by lime. And, the zeocarbon was possible for reuse and regeneration due to easiness of recovery by granule type. Ultimately, this study tries to exhibit basic data to design of one-stage for nitrogen/phosphorus simultaneous removal system zeocarbon.
The zeocarbon used in this study was produced from the Zeobuilder company (Korea Mokpo). The zeocarbon is consists of zeolite 5A(55 wt%), activated carbon (33 wt%) and calcium silicate(12 wt%). The ammonium ion exchange capacity of the zeocarbon was 1.561 meg/g. The sample was sieved with 30~35 mesh. To satisfy purpose of this study for water treatment, the surface of zeocarbon was formed functional group by acid treatment. Acid treatment was used 1 N hydrochloric acid for 2 h in room temperature. Weight ratio of the zeocarbon to hydrochloric acid was 1 to 1.5. After acid treatment, the sample was rinsed with distilled water to remove chloride ion on the zeocarbon surface and dried at 115℃ for 24 h. And, the sample was stored in a desiccator at room temperature before each set of experiments.
2.2. Design of experiments and analysis
Strength of the modified zeocarbon was investigated to see possibility for application of water treatment. In experiments for ammonium and nitrate removal, article wastewater was used 0.003 M solution as NH4NO3. The effect of the reaction temperature on nitrogen removal efficiency was evaluated.This experiment carried out at 15℃, 25℃, 35℃ and 45℃ respectively. Also, the effect of coexisting cation such as Ca2+, Mg2+ and K+ on removal of ammonium and nitrate was investigated. This study was conducted with initial cation concentrations of 10 mg/L, 30 mg/L, 50 mg/L, 100 mg/L and 150 mg/L. The experiment for phosphorus removal was conducted under presence of ammonium and nitrate. The experiment for simultaneous removal of ammonium, nitrate and phosphorus was used 0.003 M NH4NO3 solutions contained 10 mg/L as phosphorus. This experiment carried out comparatively commercial zeolite and activated carbon which was treated on equal terms to zeocarbon for a verification of objectivity about this study results.
The residual ammonium concentration was ensured using ammonia electrode (Orion 720A). The electrode was calibrated using standard ammonium solution before every analysis. Calibration is performed in a series of standards that prepared freshly from standard ammonium solution (0.1 M NH4+: Orion 951006). Nitrate and phosphorus was measured using UV/VIS spectrometer (Hach DR/4000). Crushing strength of the zeocarbon was ensured using a digital indicator (Model : FS-1010). The specific surface area and pore volume of the zeocarbon samples were measured using BET method (Micrometrics, ASAP 2010), after the zeocarbon samples were treated by degas at 250℃ for 6 h.
3.1. Physical properties of the zeocarbon
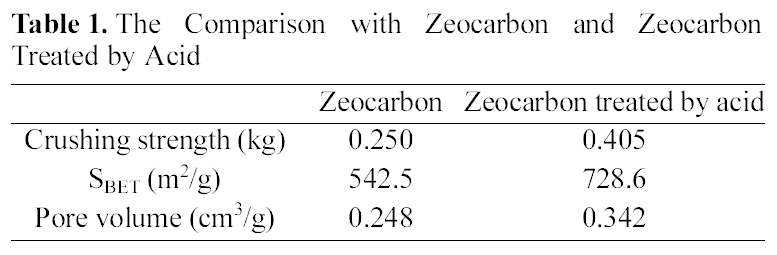
Surface modification of the zeocarbon was conducted for application of water treatment. After surface modification by acid, the crushing strength of modified zeocarbon was enhanced about 62% in comparison with the zeocarbon as shown in Table
[Table 1.] The Comparison with Zeocarbon and Zeocarbon Treated by Acid
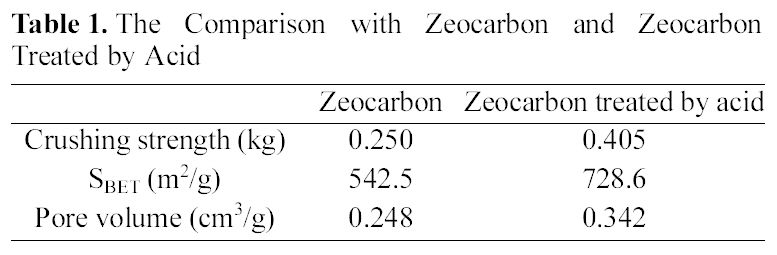
The Comparison with Zeocarbon and Zeocarbon Treated by Acid
1. This result concludes that the cause of increased strength is chemical combination of component in zeocarbon and functional groups on zeocarbon surface generated by acid treatment [4,5]. Also, the specific surface area of zeocarbon increased through acid treatment process. These reason is that had blocked some pores of activated carbon opened via acid treatment process [6]. Accordingly, the surface modified zeocarbon was suited our purpose for application of water treatment. Also, enhanced strength of the zeocarbon could be expected possibility of regeneration and reuse due to maintenance of granule form after water treatment.
3.2. Batch type experiment results
3.2.1. Characteristics of the surface modified zeocarbon
As results of preliminary experiment using the raw zeocarbon, nitrate was rarely removed. Hereupon, surface of zeocarbon was modified by acid to remove simultaneously ammonium and nitrate. As shown in Fig. 1, removal efficiency of ammonium using the surface modified zeocarbon was enhanced about 47% higher than that of the zeocarbon and removal efficiency of nitrate was enhanced about 32%. Also, removal efficiency as total nitrogen was shown a tendency to increase in 40%. These indicate that chemical adsorption by functional groups on zeocarbon was formed with the ion exchange by Ca2+ ion in zeolite and adsorption by activated carbon [7].
3.2.2. Effect of temperature on nitrogen removal
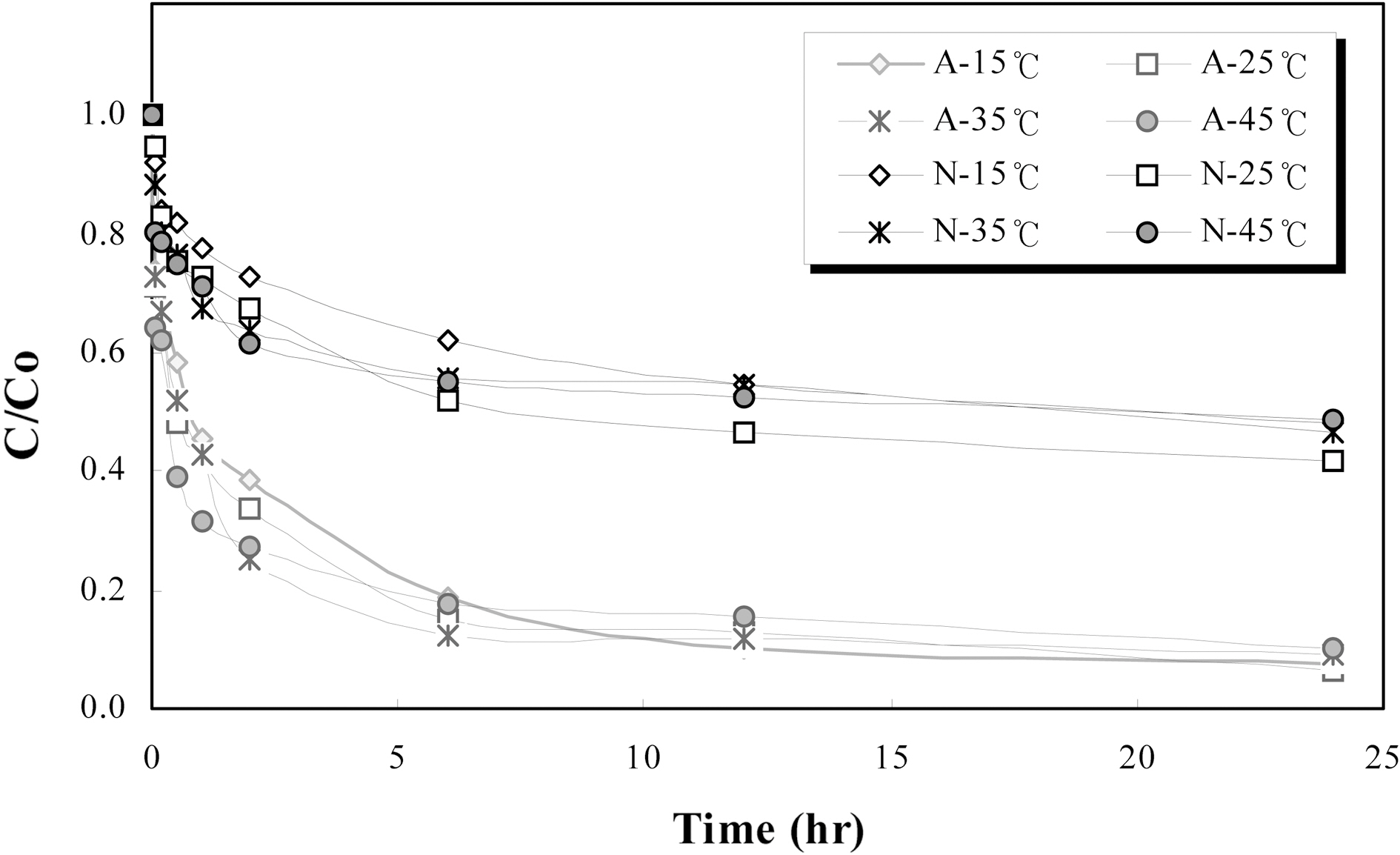
This study carried out at 15℃, 25℃, 35℃ and 45℃ respectively to investigate the effect of reaction temperature on removal of ammonium and nitrate. The results are presented in Fig. 2. In Fig. 2, the equilibrium adsorption time of ammonium and nitrate was found to be 6 h respectively. As can be seen from the Fig. 3, there is an insignificant effect of temperature on ammonium and nitrate removal under the test condition applied in this study. That is, removal efficiencies of ammonium and nitrate were constant comparatively according to temperature. This result could be suggested that the surface modified zeocarbon was not influenced about reaction temperature on ammonium and nitrate removal. Therefore, the modified zeocarbon will be possible to application throughout the year.
3.2.3. Effect of coexisting cation on nitrogen removal
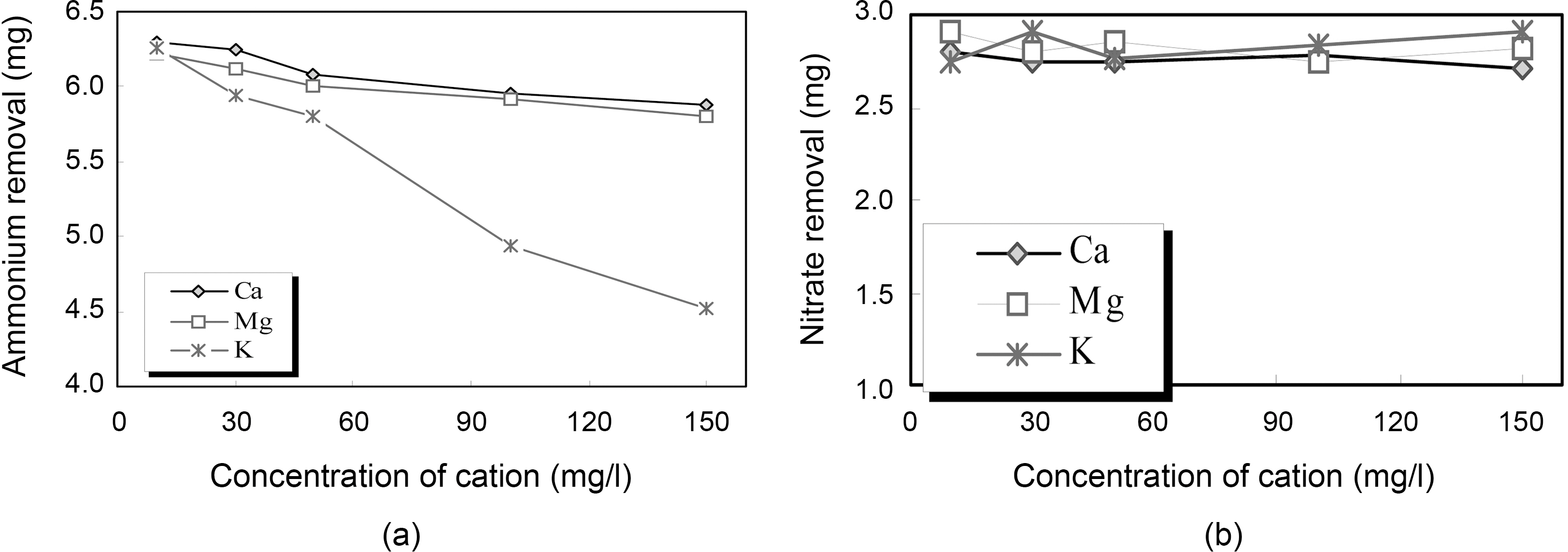
To evaluate the effect of coexisting cation such as Ca2+, Mg2+ and K+ on removal of ammonium and nitrate, the studies were conducted with initial cation concentrations of 10 mg/L, 30 mg/L, 50 mg/L, 100 mg/L and 150 mg/L. The experimental data of ammonium and nitrate removal at a fixed zeocarbon quantity (20 g/L) are presented in Fig. 3. As shown in Fig. 3 (a), existence of cations such as Ca2+, Mg2+
was hardly influenced on ammonium removal. However, the removal efficiency of ammonium decreased with the increase of K+ ion concentration. This could be explained by the fact that increasing K+ ion concentration in the solution might have hindered the ion exchange of ammonium.Accordingly, the ammonium ion selectivity was reduced by K+ ion. And, the order of strength to reduce the ammonium exchange was K+>Mg2+>Ca2+[8,9].
Removal of nitrate under coexisting cation was shown in Fig. 3(b). As shown in Fig. 3(b), the removal efficiency of nitrate was no difference according to increased concentration of cations such as Ca2+, Mg2+ and K+. That is, there is an insignificant effect of cation on nitrate removal under the test condition applied in this study.
3.2.4. Simultaneous removal of ammonium, nitrate and phosphorus
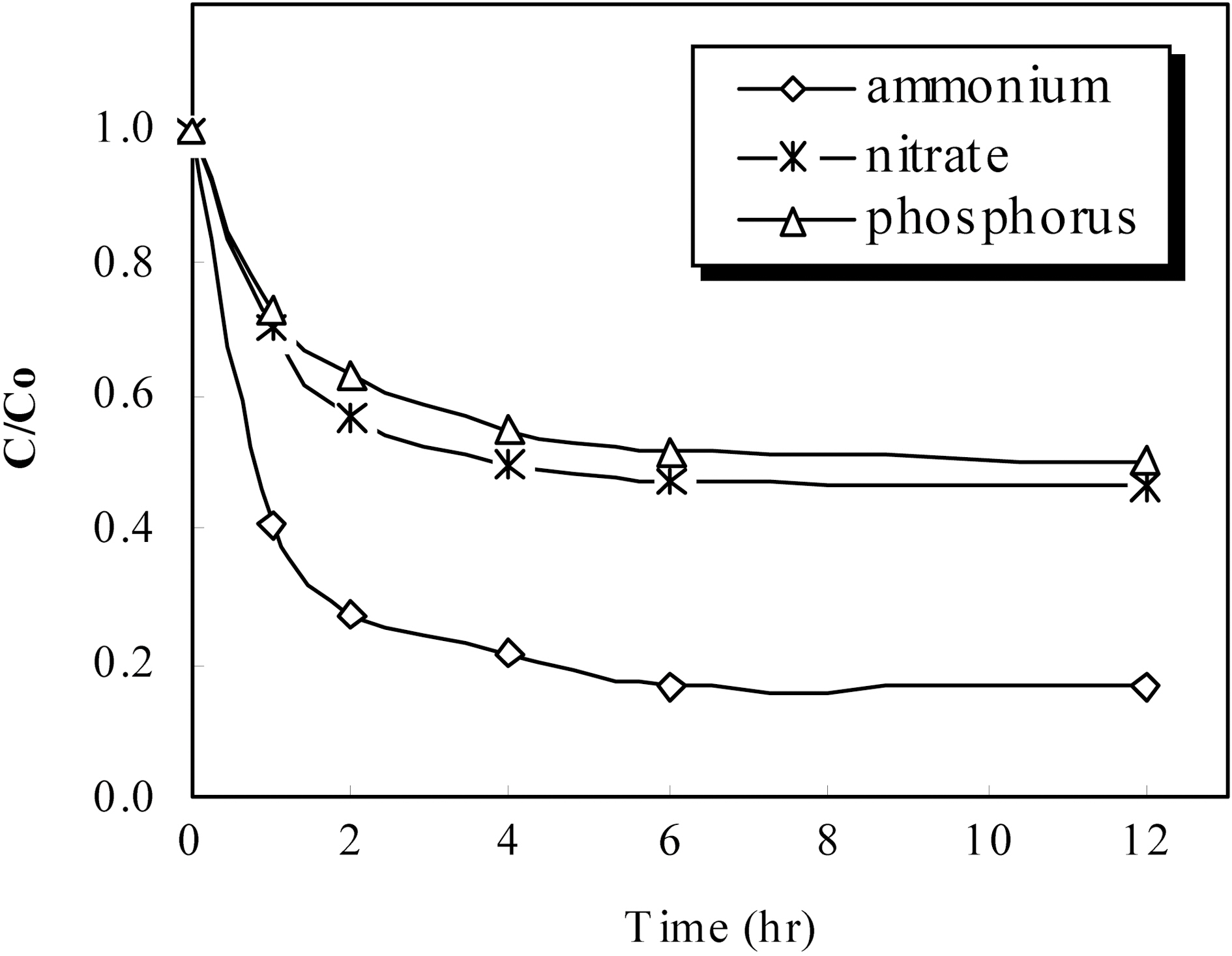
As a result of simultaneous removal of ammonium and nitrate, removal efficiency of ammonium and nitrate was investigated respectively 76% and 40% after reaction for 2 h. This result was satisfied the possibility for simultaneous removal of ammonium and nitrate. So, to evaluate the possibility for simultaneous removal of phosphorus with ammonium and nitrate removal, the experiment was progressed. In experiment for simultaneous removal of ammonium, nitrate and phosphorus, the surface modified zeocarbon 20 g put into 0.003 M NH4NO3 article waste water contained 10 mg/L as phosphorus. And, we were ensured removal efficiency of ammonium, nitrate and phosphorus according to reaction time. This result was presented in Fig. 4. As a shown in Fig. 4, removal efficiency of phosphorus was obtained about 35% after reaction for 2 h. Besides, existence of phosphorus in solution was not influenced on removal efficiency of ammonium and nitrate. This result could be expected that the possibility for simultaneous removal of ammonium, nitrate and phosphorus is enough. Further experiments must be carried out simultaneous removal of nitrogen and phosphorus connected with the zeocarbon.
[Fig. 4.] Sorption kinetics of ammonium nitrate and phosphorus using the surface modified zeocarbon.
3.2.5. Comparison of removal characteristics of commercial absorbents
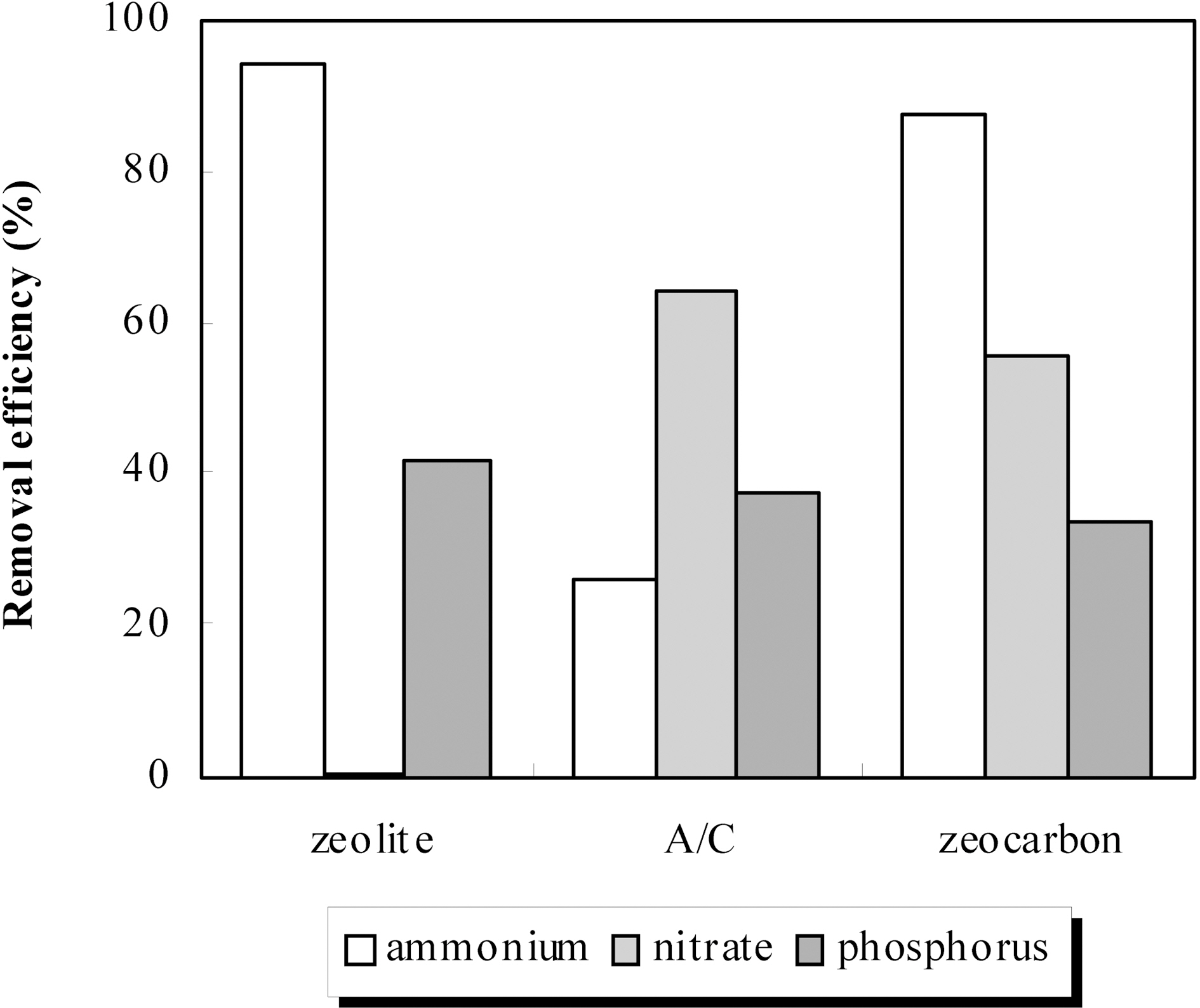
To objectification of our results, the experiment for removal of ammonium, nitrate and phosphorus was carried out comparatively commercial zeolite 5A (specific surface area:550m2/g) and activated carbon (specific surface area: 850m2/g) which was treated on equal terms to the zeocarbon. The reason that the commercial samples were pretreated was as following the results of preliminary test. As a result of preliminary experiment, removal efficiencies of ammonium, nitrate and phosphorus using the modified absorbents by acid were respectively better than that of original one. As shown in Fig. 5, the zeolite was shown high removal efficiency of ammonium like widely known but has no removal efficiency of nitrate [10,11]. In case of the activated carbon, removal efficiency of nitrate was better than other absorbents due to predominant adsorption capacity by high specific surface area but removal efficiency of ammonium was the lowest. In opposition to, the modified zeocarbon has high removal efficiency compared with the commercial absorbents about simultaneous removal of ammonium and nitrate. This result was explained that the zeocarbon has ion exchange capacity and adsorption capacity synchronously because the zeocarbon is mixture of zeolite and activated carbon. Removal efficiency of phosphorus has no difference as a function of absorbents contrary to ammonium and nitrate. And, removal efficiency of phosphorus was not exerted influence on removal efficiency of ammonium and nitrate. Moreover, removal of phosphorus was possible to 35% by the zeocarbon. This indicates that the possibility for simultaneous removal of nitrogen and phosphorus is very bright. Accordingly, the surface modified zeocarbon was very effective for simultaneous removal of ammonium, nitrate and phosphorus. And, the surface modified zeocarbon regard as appropriate to application for real process.
The zeocarbon was possible to application for water treatment due to maintenance of its strength after surface modification. This feature could be expected that the zeocarbon has economical efficiency because of easiness of reuse and regeneration. In experiment for simultaneous removal of ammonium and nitrate, the removal efficiency using the surface modified zeocarbon showed about two times higher than that of the zeocarbon and the dependences of temperature and coexisting cation were found to be minimized. These results indicate that the surface modified zeocarbon was effective for simultaneous removal of ammonium and nitrate. Also, presence of phosphorus was not exerted influence on nitrogen removal and removal efficiency of phosphorus could be obtained about 35%. Accordingly, the possibility for simultaneous removal of ammonium, nitrate and phosphorus using the surface modified zeocarbon were sufficient.
In conclusion, the surface modified zeocarbon was very effective for simultaneous removal of ammonium, nitrate and phosphorus from aqueous solution. On the basis of these experiment results, we may forecast commercial availability of zeocarbon for advanced water treatment processes. Moreover, our results present the basic data for the design of one-stage nitrogen/phosphorus simultaneous removal system.