The synthesis of C60@MWCNT was carried out at room temperature (~25oC) from arc-discharge prepared Multi-wall carbon nanotubes (MWCNTs). They were oxidized and acid treated for tube opening. Then C60 molecules were encapsulated into MWCNTs by wetting them with C60-toluene solution for several minutes followed by ultrasonification. C60@MWCNT was cleaned by pure toluene to remove any excess C60. C60@MWCNT was characterized by electron microscopy, which showed large scale filling of C60 into MWCNTs. It was observed that the mechanism of insertion of C60 into MWCNTs may be due to the capillary suction at the opening ends of MWCNTs.
Fullerene encapsulation into single-wall carbon nanotubes (SWCNTs) has generated considerable interest in recent years after the discovery of C60@SWCNTs [1] because inserting fullerenes into the inner space of carbon nanotubes (CNTs) is found to bring significant changes to their electronic states, charge transport, and one-dimensional (1D) quantum characteristics [2-4]. Scanning tunneling spectroscopy studies have shown that fullerenes or metallo-fullerenes encapsulated in semiconducting SWCNT significantly modify their electronic structures due to a significant change in their band structure and affect phenomena such as even-odd effect, shell-filling in two spin-degenerate electronic states, and Kondo effect [4-6]. Recently, a single wall carbon nanotube based nanocontainer is designed for hydrogen storage, in which a C60 ‘‘peapod’’ at the cap section of the nanotube serves as a molecular valve [7].
Fullerene encapsulation was reported mainly with SWCNTs. We first studied the filling of MWCNT with C60 [8], where encapsulation of C60 into MWCNT was performed by vapor phase at 650oC at the pressure of 200 μPa. Such a high vacuum was must as we could not find any C60 into MWCNTs at low vacuum. However, filling at a high vacuum is time consuming and needs special equipment to obtain desirable vacuum and it is not available at many conventional laboratories. Therefore, in this work,room temperature (~25oC) liquid phase encapsulation of C60 inside MWCNTs is presented. High-resolution TEM images clearly showed large scale filling of C60 into MWCNTs with high yield.
High quality multi-wall carbon nanotubes (MWCNTs) were obtained from Chemapol Industries Bombay (India). MWCNTs are synthesis under helium atmosphere (~700 mbar) in the arcdischarge apparatus, which consists of a two-wall system [9]. The outer area of the inner wall is equipped with cooling pipes for efficient cooling of SWCNTs soot. The cathode consists of ultra pure graphite rod (15 mm diameter, 15 mm length). The anode consists of ultra pure graphite rod of outer diameter 6 mm (10 cm long). A current of 60 A was passed between anode and cathode to produce arc-discharge plasma. The distance between anode and cathode was kept constant at 2 mm and voltage of 30 V was maintained during the consumption of anode and subsequent production of SWCNTs. The duration of this process was kept 120 seconds.After the arc-discharge experiment, MWCNTs were extracted from the cathode deposit. The preparation of the C60 is similar to MWCNT except that the helium pressure is kept at ~20 mbar.
All the samples were characterize by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM was carried out on a Philips (FEI XL30 LaB6, 30 kV) equipped with EDAX detector. HRTEM, FFT and selected-area electron diffraction measurements and EDX elemental analysis were carried out on a Philips Tecnai 20 S-TWIN (200 kV) field emission HRTEM equipped with a post specimen aberration corrector. The diameter of a C60 is ~0.7 nm [10], whereas the internal diameter of a MWCNT was in the range of 5~10 nm as ascertained by TEM images.
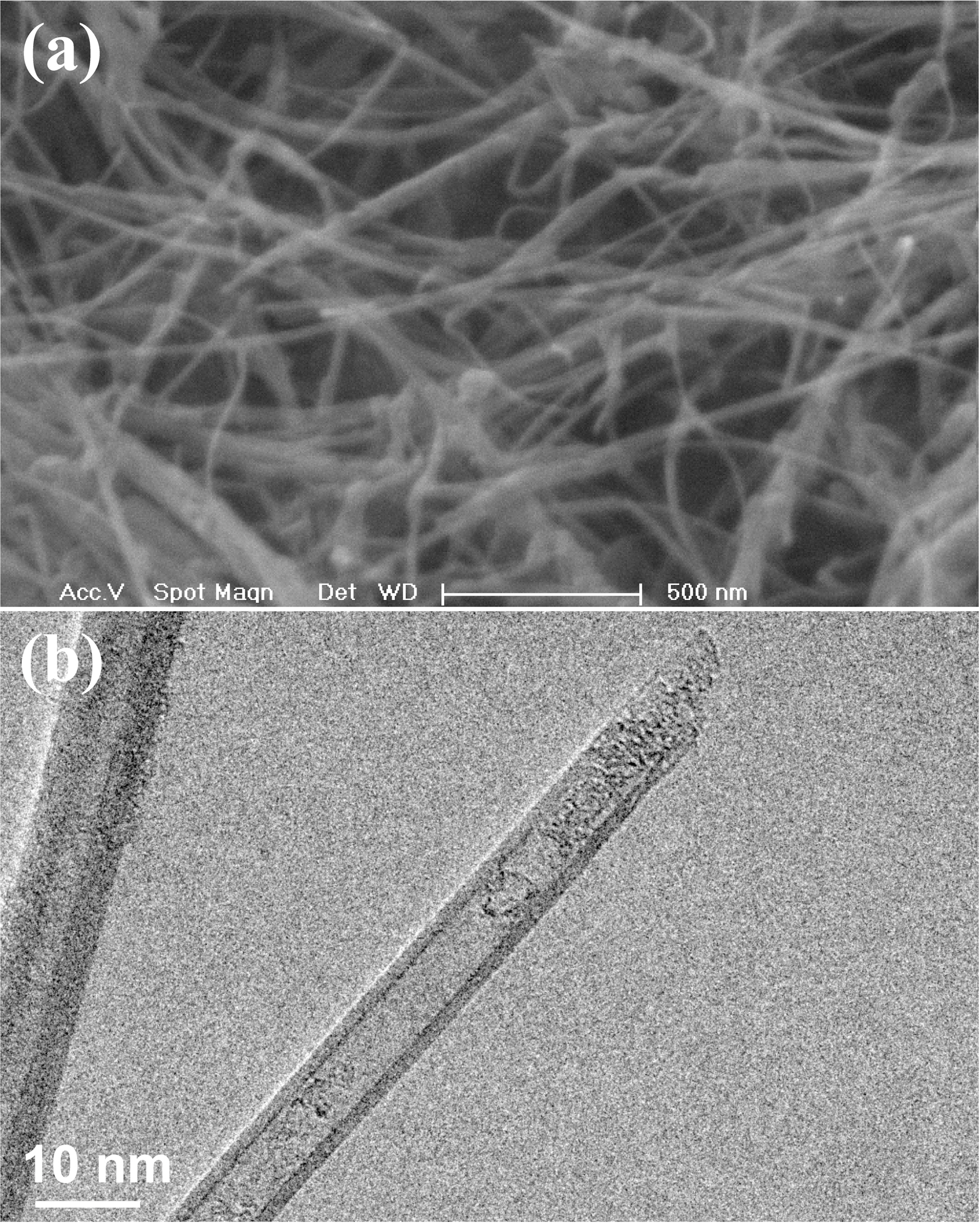
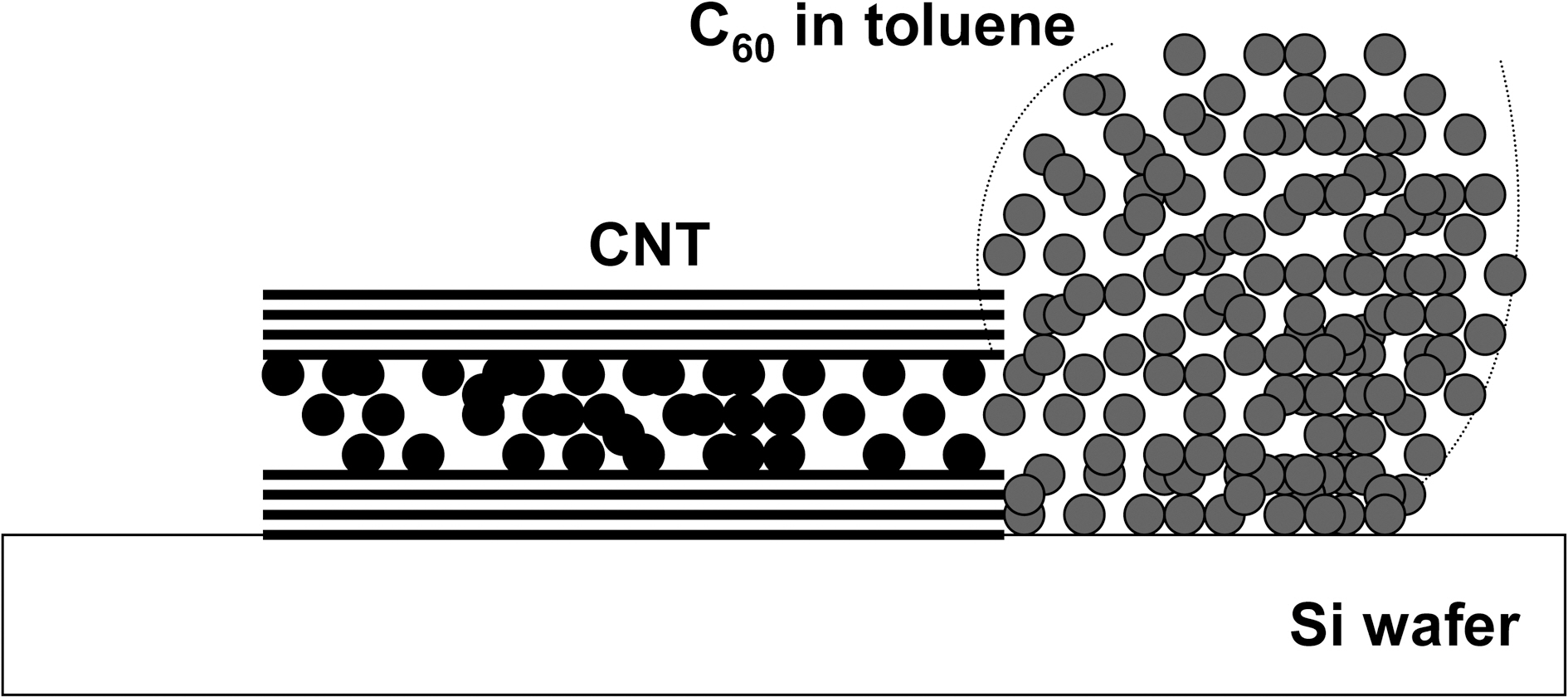
Fig.1(a) shows scanning electron microscopy (SEM) image of MWCNTs. The opening of the nanotubes was performed in a three step process. Initially the nanotubes were oxidized in air at 700oC for 10 minutes. The heating and cooling rates were rate were 20oC/min [11]. Tube opening by oxidation in air results in an only very small amount of open nanotube(~4%). In the second step, 65% nitric acid was added and the mixture was refluxed for 4.5 h at 125oC after which MWCNTs were washed with distilled water several times until the pH is close to 7 [12]. In the final step the nanotubes were oxidized again at a temperature of 600oC in air for 10 minutes onto a silicon wafer to remove any traces of acids, after which it was cooled down to room temperature at 20oC/min. The silicon wafer was used because it provides a smooth surface and has a very high meting point. TEM images showed an average of 30% uncapped nanotube ends. Fig.1(b) shows transmission electron microscopy (TEM) image of an uncapped MWCNT. The C60-toluene solution was prepared by adding commercial C60 (99.9% purity) to toluene in 1 mg ml-1 ratio. Fig. 2 depicts the experimental set up for the synthesis of C60@MWCNT [13]. C60-toluene was added on to the MWCNTs drop-wise so that the entire sample is covered. After the solution is dried-up, the process
was again repeated several times. Afterward, the sample was transferred to C60-toluene solution and sonicated for 1 hour.Finally, the obtained sample was sonicated in toluene for 1 hour to remove excess C60 and then air dried. Further drying was done at 150°C for 2 h in a moderate argon flow.
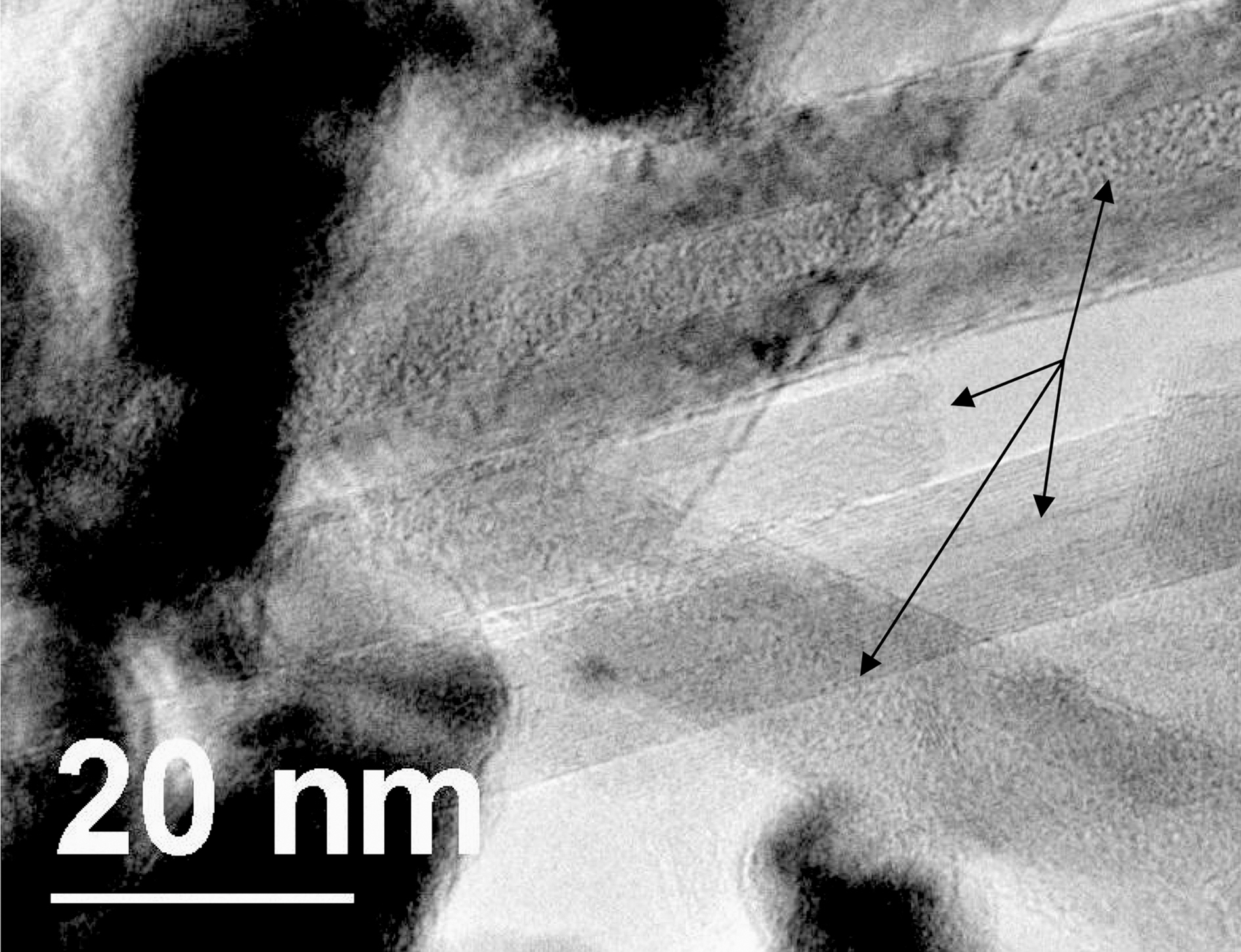
Fig. 3(a), (b) depicts the process of filling of C60 into MWCNT. It has been shown theoretically and experimentally that the procedure adopted in Fig. 2 results in the filling of carbon nanotube through the combined action of capillary forces and evaporation [13,14]. Fig. 3(a), (b) suggests that the filling of C60 into MWCNTs is most likely due to the same mechanism. Fig. 3(b) shows the negative image of the Fig. 3(a) to clearly identify the extent and location of C60(which are bright spots).
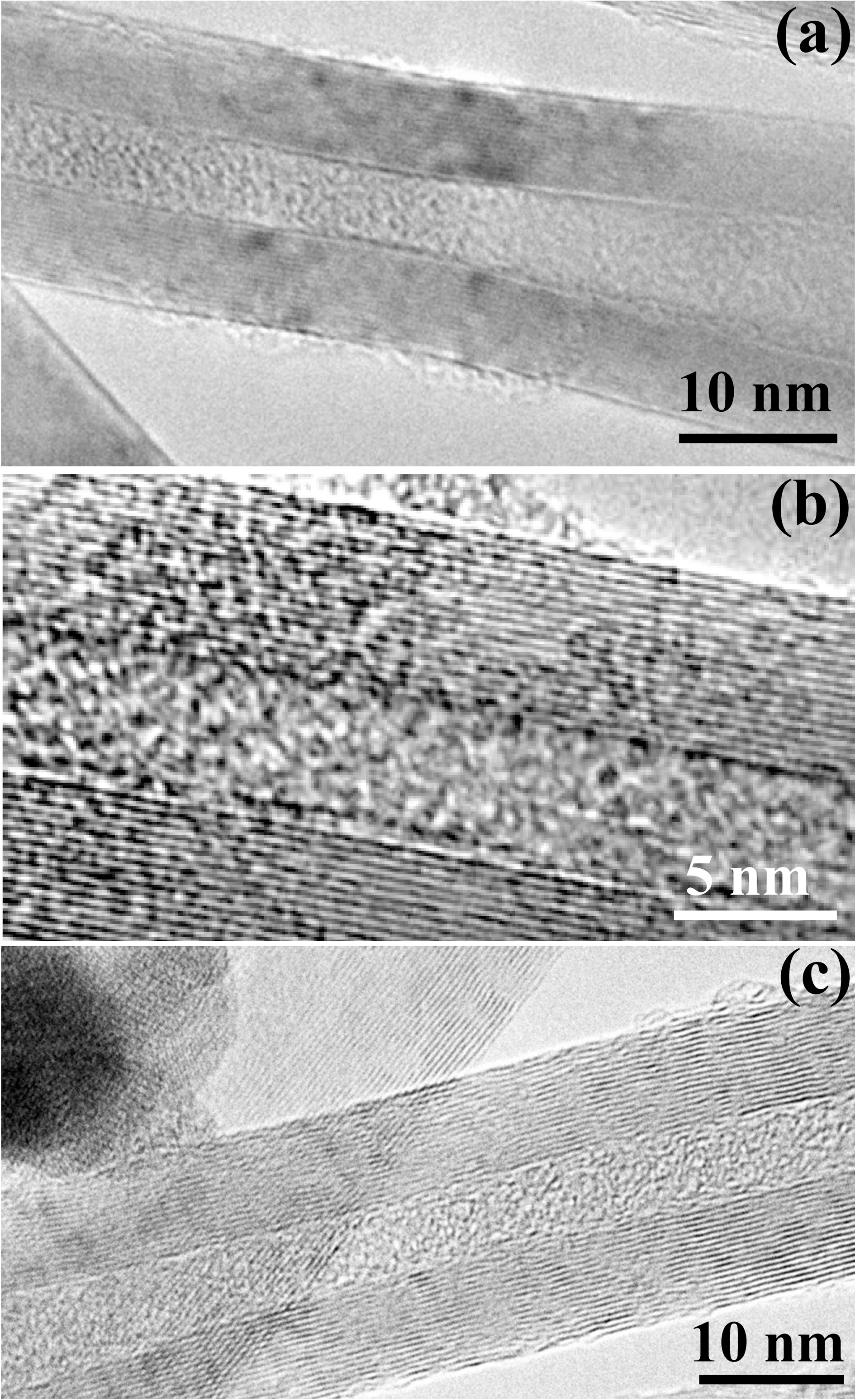
Fig. 4(a)~(c) shows TEM images of C60@MWCNT, where C60 completely fills the MWCNTs. The diameter of a C60 molecule is 0.7 nm and graphitic van der Waals separation is 0.3 nm [10]. The arrangement of C60 molecules in a MWCNT depends upon its diameter. Generally, there are two kinds of arrangements. If the inner diameter of a nanotube is only a few times larger than the diameter of C60,it tend to form zigzag chains which were also reported for C60 in BN nanotubes [15]. For larger diameters, C60 consists of irregularly arranged clusters. In the present case, due to a large inner diameter of MWCNTs, C60 clusters are irregularly arranged [16]. Fig. 4(a) shows a deformed MWCNT, where, in the smaller diameter region C60
molecules are irregularly arranged and for larger diameter region, the TEM image becomes fuzzy due to the continuous movement of C60 clusters. In addition, the fullerenes possibly begin to coalesce under the intense electron beam irradiation [1]. For the diameter of C60 molecule of 0.7 nm and graphitic van der Waals separation of 0.3 nm, a 5 nm(inner diameter) MWCNT can accommodate around five C60 molecule in a plane but π×(diameter/2)2 along the cross section, the TEM images (Fig. 4) show a large number of densely packed C60 clusters overlapping each other because TEM picture is only a two-dimensional projection. One can observe that C60 molecules also tend to form zigzag chains [17,18] and this zigzag arrangement can freely rotate around the long tube axis. Fig. 4(a) shows a low resolution TEM image where a large number of C60@MWCNT is shown. In conclusion, the present method can provide scope for a large scale filling of MWCNTs.