As a type of high-performance inorganic fiber, carbon fiber has lots of good superiorities such as specific strength, high modulus, lightfastness, corrosion resistance, fatigue, creep resistance, electrical conductivity, excellent heat transfer performance, and etc. [1,2]. When temperature is as high as 2000oC, carbon fiber is the only material that the strength does not fall in an inert environment. It is well known that the properties of high performance polyacrylonitrile (PAN) based carbon fiber are determined strongly by the nature of precursor, and preparing high molecular weight polymer is one of the effective ways to obtain high strength fiber [3-6].In general, a second monomer such as acrylic ester and a third monomer such as itaconic acid were added to monomer in order to increase the molecular weight of PAN in solution polymerization.
Recently, precipitation polymerization is increasing the interests in the preparation of high molecular weight PAN due to the advantage of small chain transfer reaction than solution polymerization in using a second monomer [7-10].Bahrami [11] reported the effect of H2O/dimethyl formamide(DMF) on the conversion rate and intrinsic viscosity during precipitation polymerization using acrylonitrile and acrylic acid as monomers and H2O and DMF as mixed solvent system. Wang [12] studied the effect of monomer concentration,initiator concentration, ratio of H2O/dimethyl sulfoxide (DMSO) on the precipitation polymerization, and suggested that precipitation polymerization was more effective than solution polymerization in preparation of high performance carbon fibers. Zhang [13] also studied the effect of precipitation polymerization conditions on the conversion rate using H2O/DMSO mixture solution.Wang [14] reported the relationship between apparent activation energy and the ratio of H2O/DMSO. However,there are few studies on the relationship between molecular weight increase of PAN and H2O/DMSO ratio, especially relating to the theoretical mechanism of PAN molecular weight increase.
Therefore, the purpose of this study is experimental and theoretical investigations of PAN molecular weight increase in precipitation polymerization as functions of H2O/DMSO ratio and intrinsic viscosity. To obtain the purpose, i)precipitation polymerization of acrylonitrile was carried out by changing the solvent ratio of H2O/DMSO at different temperature, ii) molecular weight and intrinsic viscosity of the obtained polymer were measured in DMF solution, and iii) model mechanism of precipitation polymerization was suggested in relation between molecular weight increase of PAN and H2O/DMSO ratio.
Acrylonitrile (AN, Beijing Chemical Reagents Co.) was freed from inhibitors by distillation at 76~78oC before polymerization. α,α’-azobisisobutyronitrile (AIBN) was purified by recrystallization. Dimethyl sulfoxide (DMSO)and deionized water (H2O) were used as polymerization solvents. Dimethyl formamide (DMF) solution was used to compare the intrinsic viscosity of the polymer with that of DMSO solution.
2.2. Precipitation polymerization
Precipitation polymerization of AN was carried out in a 1.0 L three-necked glass reactor by adding 0.3 L different H2O/DMSO ratio from 10/90 to 80/20 (wt/wt) as solvent to 0.1 L AN for 2 h at 50, 55, 60 and 65oC under pure nitrogen atmosphere. After polymerization, enough distilled water was added into the reactor to precipitate the produced polymer. The precipitated polymer was washed with distilled water and ethanol successively, filtered with Buchner funnel,and dried under vacuum at 40oC till a constant weight was obtained.
2.3. Intrinsic viscosity measurement
Intrinsic viscosity [η] of the obtained polymers was measured in DMF solution using an Ubbelohde viscometer in a water bath at constant temperature of 35 ± 0.1oC following the Kashyap’s method [15]. The viscosity average molecular weight Mη was calculated from the following equation [16]:
Fig. 1 shows the SEM images of PAN obtained from H2O/DMSO ratio of 10/90 and 80/20 in precipitation polymerization at 55oC for 2 h. The morphology of PAN obtained from small ratio of H2O (10/90) shows loosely aggregated large particles. While that of obtained from 80/20 shows big spheres composed of tightly aggregated small particles. These images suggested that there might be immense number of micro-droplet monomers in 80/20 ratio solvent which was divided by H2O at the beginning of precipitation polymerization. And these immense micro-droplet monomers proceeded the precipitation polymerization, then generated plenty of precipitation particles, finally resulted in the
increase of intrinsic viscosity and viscosity average molecular weight. The size and shapes of aggregation were depended on the H2O/DMSO ratio, and higher molecular weight PAN precursor can be obtained from increasing the H2O/DMSO ratio.
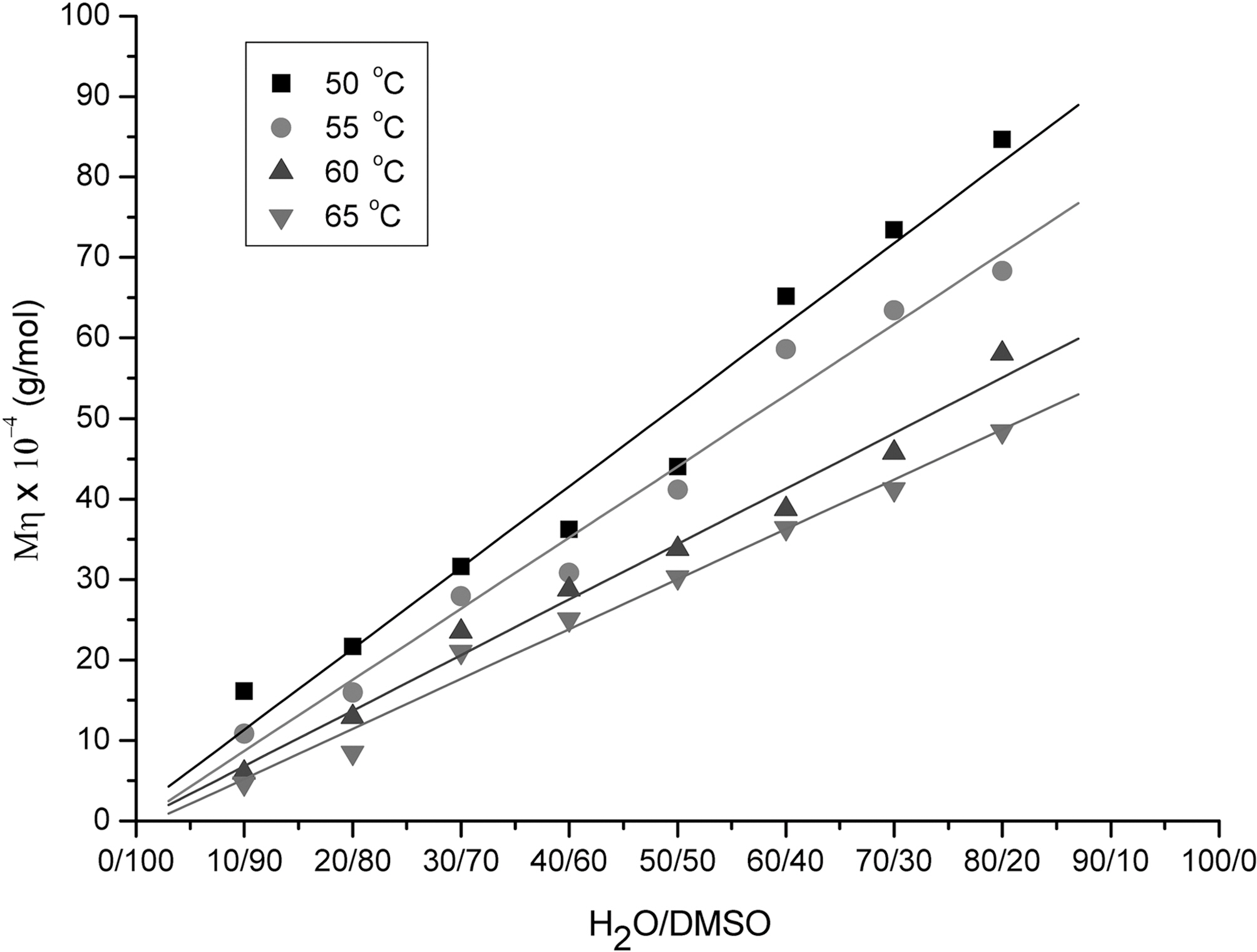
Fig. 2 shows the viscosity average molecular weights of PAN as a function of H2O/DMSO ratio obtained at 50, 55,60 and 65oC. The viscosity molecular weight linearly increased as the increase of H2O/DMSO ratio from 10/90 to 80/20. This was because the number of micro-droplet monomer increased as the increase of H2O/DMSO ratio,and the increased micro-droplet were aggregated until they became big precipitation particles, resulted in the molecular weight increase, which made the solution more turbid and viscous. Wang [12] and Zhang [13] have reported the similar results on the effect of H2O/DMSO ratio. On the other hand, the viscosity average molecular weight decreased as the increase of reaction temperature at the fixed ratio of H2O/DMSO. This was believed high
reaction temperature promoted the suspension of microdroplet rather than particles aggregation, resulted in the decrease of viscosity.
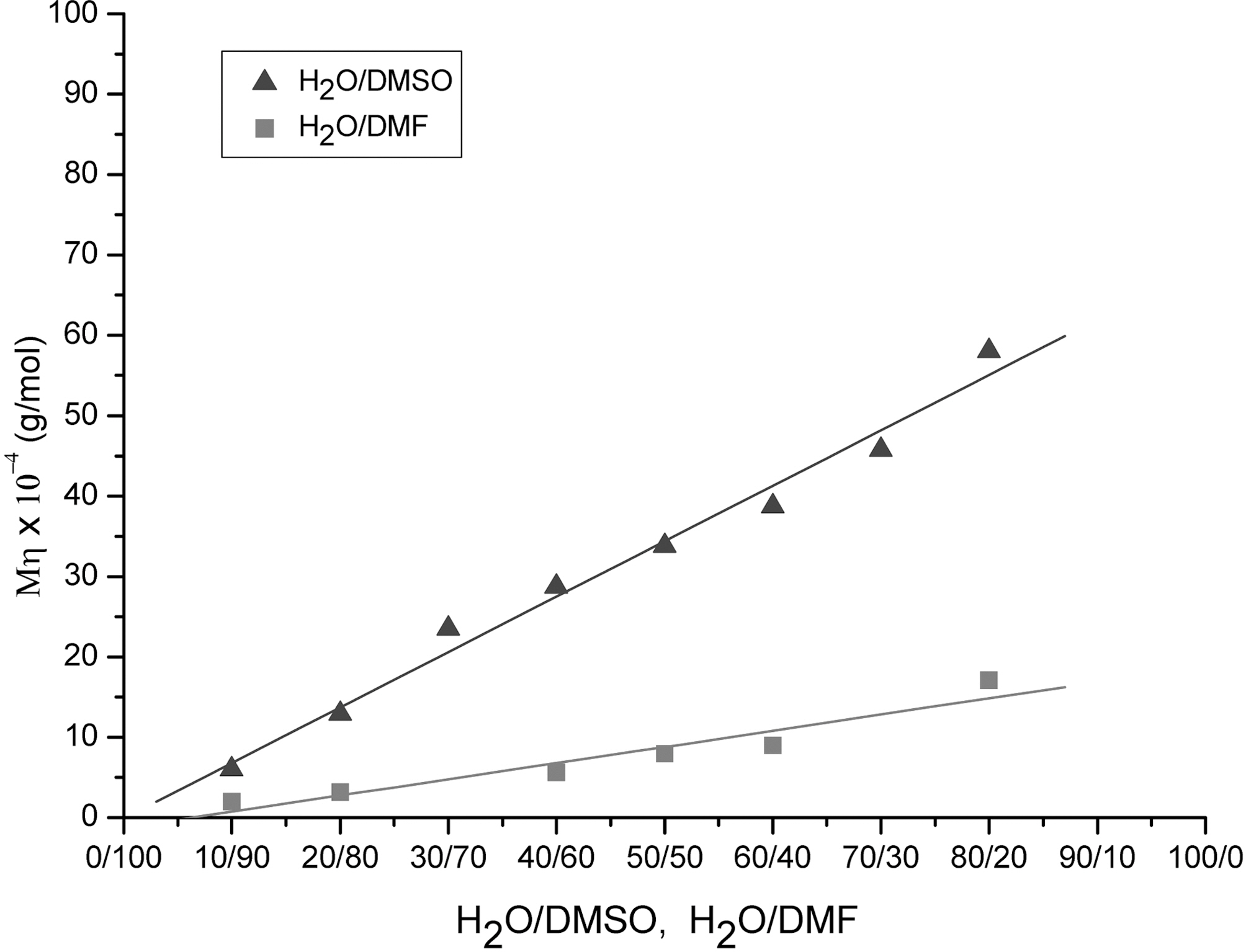
Fig. 3 shows the viscosity average molecular weights of the PAN as a function of H2O/DMSO and H2O/DMF ratio obtained from precipitation polymerization at 60oC. The viscosity average molecular weight of H2O/DMSO solvent was 4.4 times larger than that of H2O/DMF solvent. Bahrami[11] reported very similar data to those of our H2O/DMF shown in Fig. 3. The higher value of H2O/DMSO system was due to the decreased chain transfer effect of the DMSO.The chain transfer constant of water is almost zero and that of DMSO is much less than DMF [10]. Therefore, H2O/DMSO solvent system is far better than H2O/DMF system for the precipitation polymerization to obtain increased molecular weight of PAN.
Based on the experimental results, summation of two mechanisms, particle-particle aggregation and particleradical attachment can be suggested in the formation of the increased molecular weight PAN.
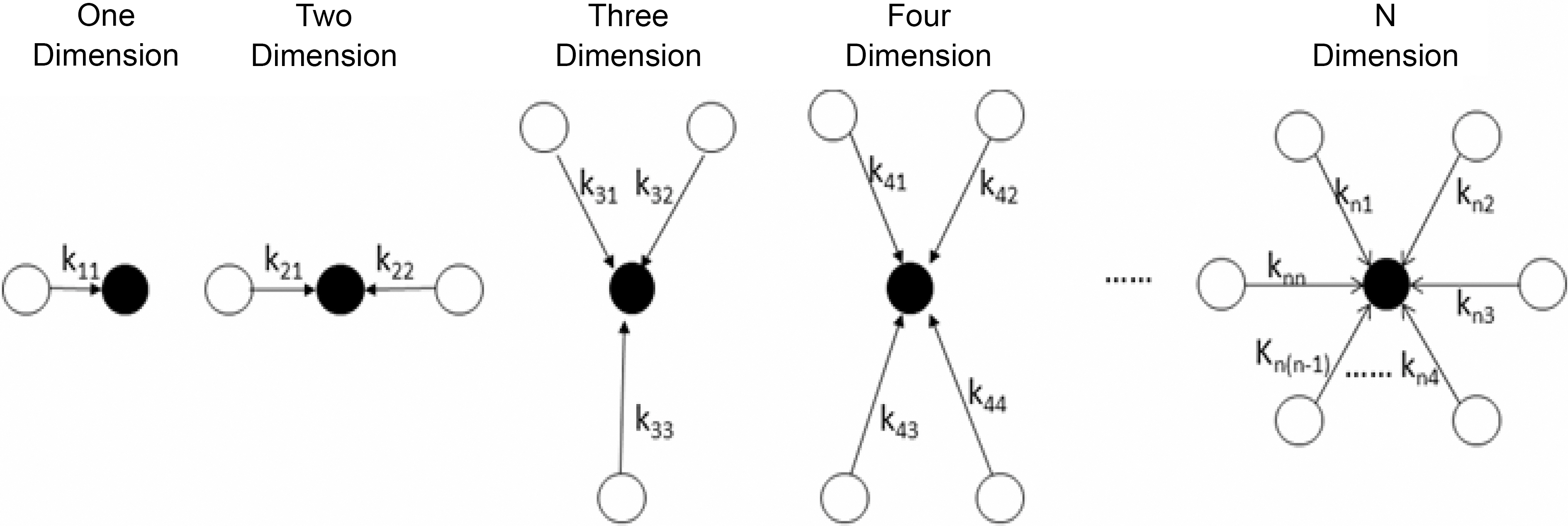
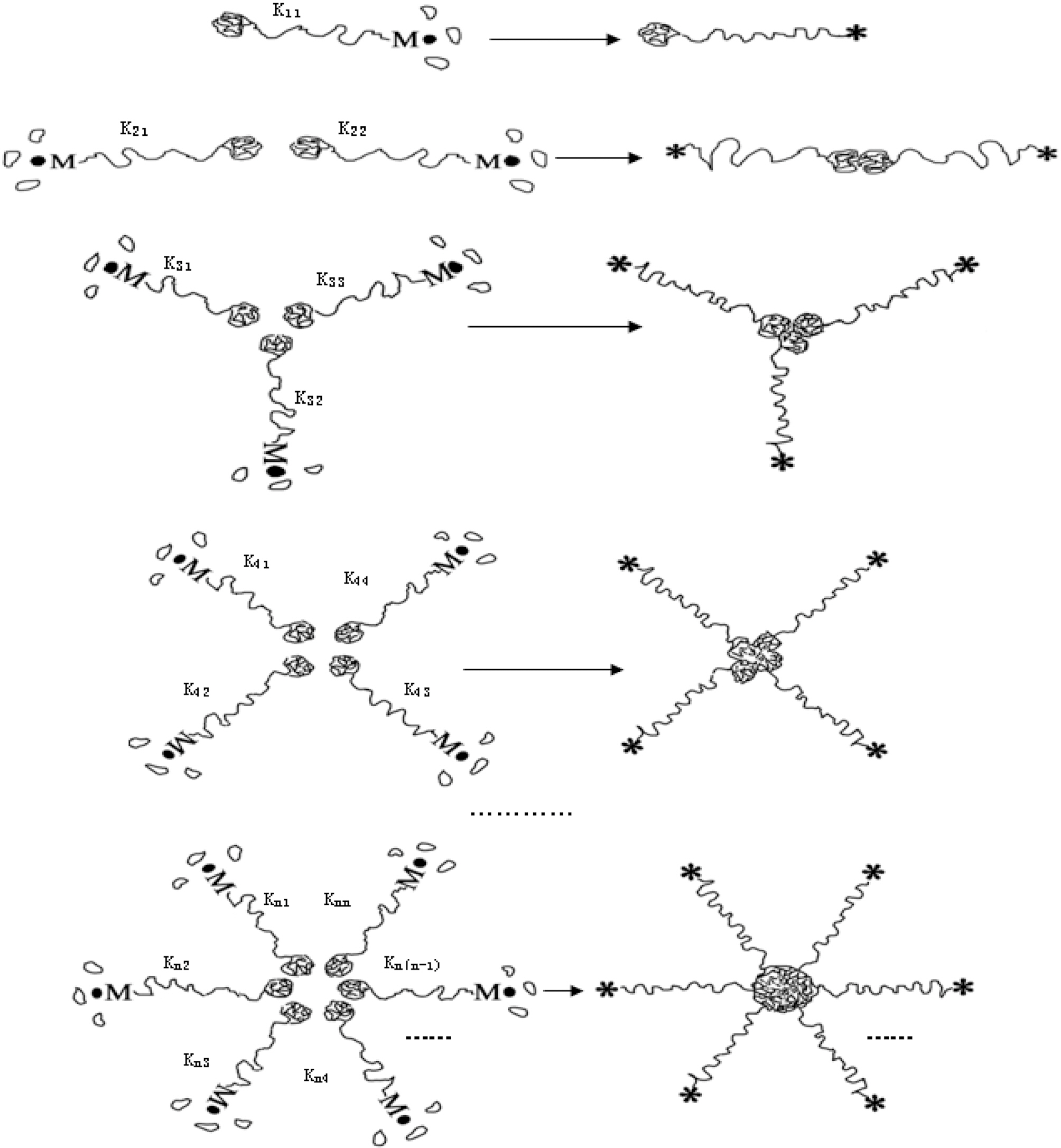
Mechanism I was regarded as a particle-particle aggregation.In the precipitation polymerization of acrylonitrile, the obtained polymer was not dissolved in the H2O/DMSO mixture solution. Once it was generated, it became nascent particle and suspended in the solution. As the core, the nascent particle aggregated with other particles until it became a larger particle in the precipitation polymerization.This process can be regarded as n-dimensional aggregation as shown in Fig. 4.
The number of micro-droplet monomer increased as the increase of H2O/DMSO ratio due to the division of droplet by H2O, which made the solution more turbid and viscous. From this consideration, the following equation can be proposed.
Where, [P] is the concentration of the nascent precipitation particle, and following hypothesis can be suggested.
Where,
i : dimension,
j : serial number of particles.
When the nascent precipitation particle aggregated with other two particles,
When the nascent precipitation particle aggregated with other three particles,
When the nascent precipitation particle aggregated with other n particles,
If there was no particle to aggregate, F(P) will be proportional to [P]0, and expressed as the following equation.
Therefore, all the aggregated nascent particles will be the summation of
If each nascent precipitation particle has same ability for precipitation and kij is the function of temperature, it can be regarded to kij=kji=k00=k11= …knn, and the equation (8)can be simplified as follows.
The equation is exactly part of the Taylor series expansion,so the equation (9) can be changed as the following form:
Therefore, the total aggregated number of nascent precipitation particles is
Where Q is the quantity of precipitates and
overall aggregation rate constant.
Mechanism II was regarded as particle-radical attachment. When the weight ratio of H2O/DMSO was small, the number of nascent precipitation particle will be limited. The propagation radical (
Based on the physical concept above, it can be put forward a model similar to the previous. When the nascent particle attached to one other particle,
Where,
i : dimension,
j : serials number of particles.
When the nascent particle reacted with two other particles,
When the nascent particle attached to three other particles,
When the nascent monomer particle attached to n other particles,
If there is no monomer particle for attachment,
Therefore, all the attached nascent monomer particles will be the summation of F(M?)1+
If each first born particle has same ability for precipitation and
The equation (18) was exactly part of the Taylor series expansion, so it can be changed as the following form:
The total attached monomer of nascent precipitation particles are
Where Q is the quantity of precipitates and
Taking logarithm on both sides of the equation:
The viscosity average molecular weight can be calculated by the summation of (2) and (22), and the final equation can be suggested as follows.
Where K3 is inversely proportional to K2 and K4 = K1/K2
From (23), it can be found that there is a linear relationship between the viscosity average molecular weight and the H2O/DMSO ratio. Therefore, the theoretical equation derived from the mechanism was well coincided with the experimental results showing the linear relationship between the viscosity average molecular weight and the H2O/DMSO ratio.
In the precipitation polymerization PAN molecular weight increase was depended on the increase of H2O/DMSO ratio due to the increased micro-droplets, which made the solution more turbid and viscous, resulted in the molecular weight increase. Using H2O/DMSO solvent system was far better than using H2O/DMF system in precipitation polymerization and obtained increased molecular weight PAN precursor because of decreased chain transfer effect of DMSO. Based on the experimental results, the increased molecular weight of PAN was regarded as the summation of two mechanisms: particle-particle aggregation and particleradical attachment. The theoretical equation derived from the mechanisms can be used to estimate the viscosity average molecular weight of the newly polymerized polyacrylonitrile (PAN) precursor.