Tannery wastewater is a powerful pollutant. It can cause severe environmental problems related to its high chemical oxygen demand (COD) together with elevated chrome concentration and deep color content [1]. Synthetic dyes are widely used in many industries including leather dyeing and paper printing. Dyes are almost invariably toxic, and additionally a visible pollutant, so their removal from effluent stream is ecologically essential. The disposal of these wastes into the environment could be harmful since they reduce light penetration and have a derogatory effect on photosynthesis. In addition, most dyestuffs are designed to be resistant to environmental conditions such as light, heat,microbial attack and also to oxidizing agents [2]. For these reasons, the biodegradation of dyes is typically a slow process [3]. Consequently, considerable research for the best available technology for dye removal from aqueous solutions has been carried out in this study.
Dyes are easily visible even in highly diluted forms,posing an aesthetic problem and are toxic to human and aquatic life [4]. The presence of carcinogens has been reported in the combined wastewater of dyeing and printing units of Udaipur [5]. They pose a problem as they may be mutagenic and carcinogenic and can cause severe damage to human beings, such as dysfunction of the kidneys, reproductive system, liver, brain and nervous system [6].
Various techniques, such as chemical coagulation using alum, lime, ferric chloride, ferric sulphate, biosorption [7],oxidation methods using chlorine and ozone [8], biological treatment, floatation [9] and adsorption, have been employed to remove dyes from industrial effluents.
Adsorption techniques for wastewater treatment have become more popular owing to their efficiency in removal of pollutants stable for biological methods. Adsorption can produce high quality water while also being a process that is economically feasible [10].
The objective of the present work was to explore the potentialities of low cost and easily available bio-wastes i.e. cow dung ash, mango stone ash, parthenium leaves ash and activated carbon for the adsorptive removal of Acid Blue 92. The effects of some operating parameters (pH, dye concentration) on the biosorption process were investigated. In addition, the applicability of the Langmuir and Freundlich isotherm models were investigated.
Adsorption studies were performed by the batch technique using biomass ash (low cost bio-waste) and activated carbon as the adsorbents without giving any pretreatment. A stock solution of the dye with a concentration of 1000 mg/ L was prepared and dilutions were made with distilled water to make different concentrations (10~100 mg/L) for the adsorption studies. A known weight of the adsorbent (1 g) was added to 50 mL of each of the above concentration in 100 mL measuring flasks. These were placed in an air thermostat for
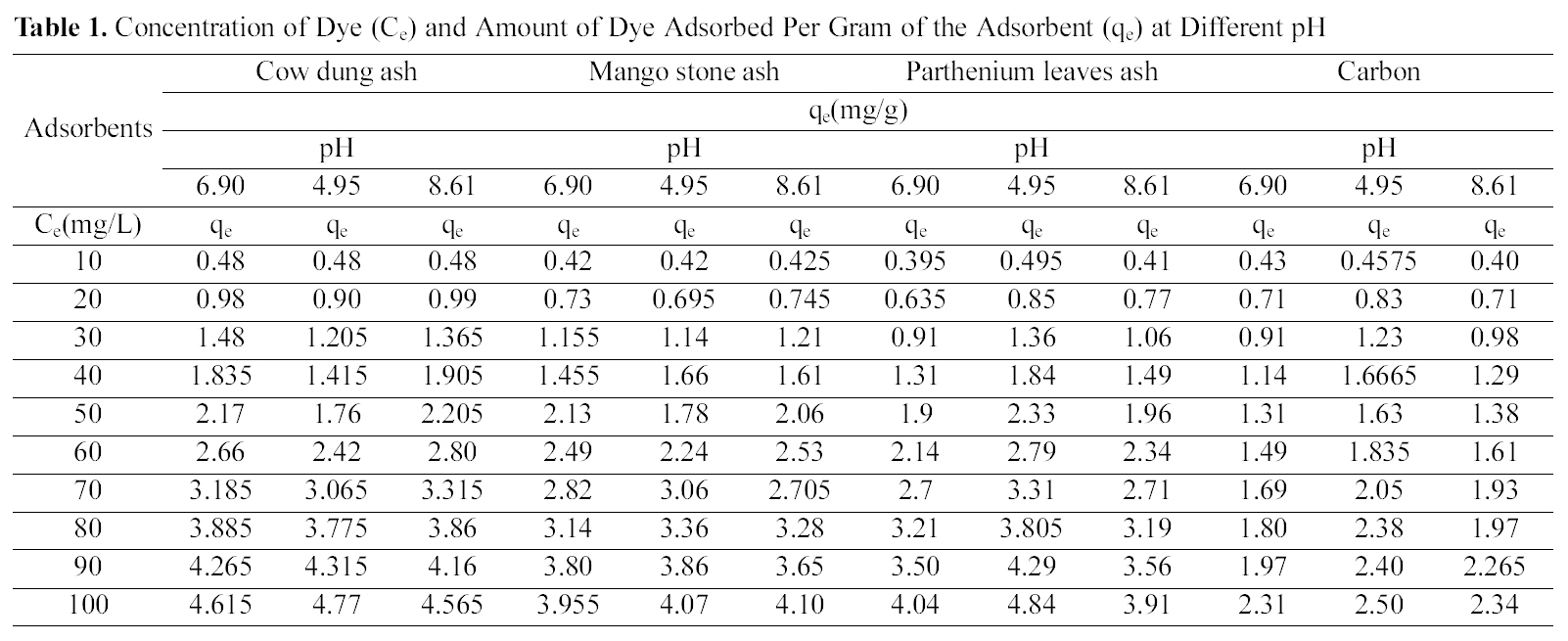
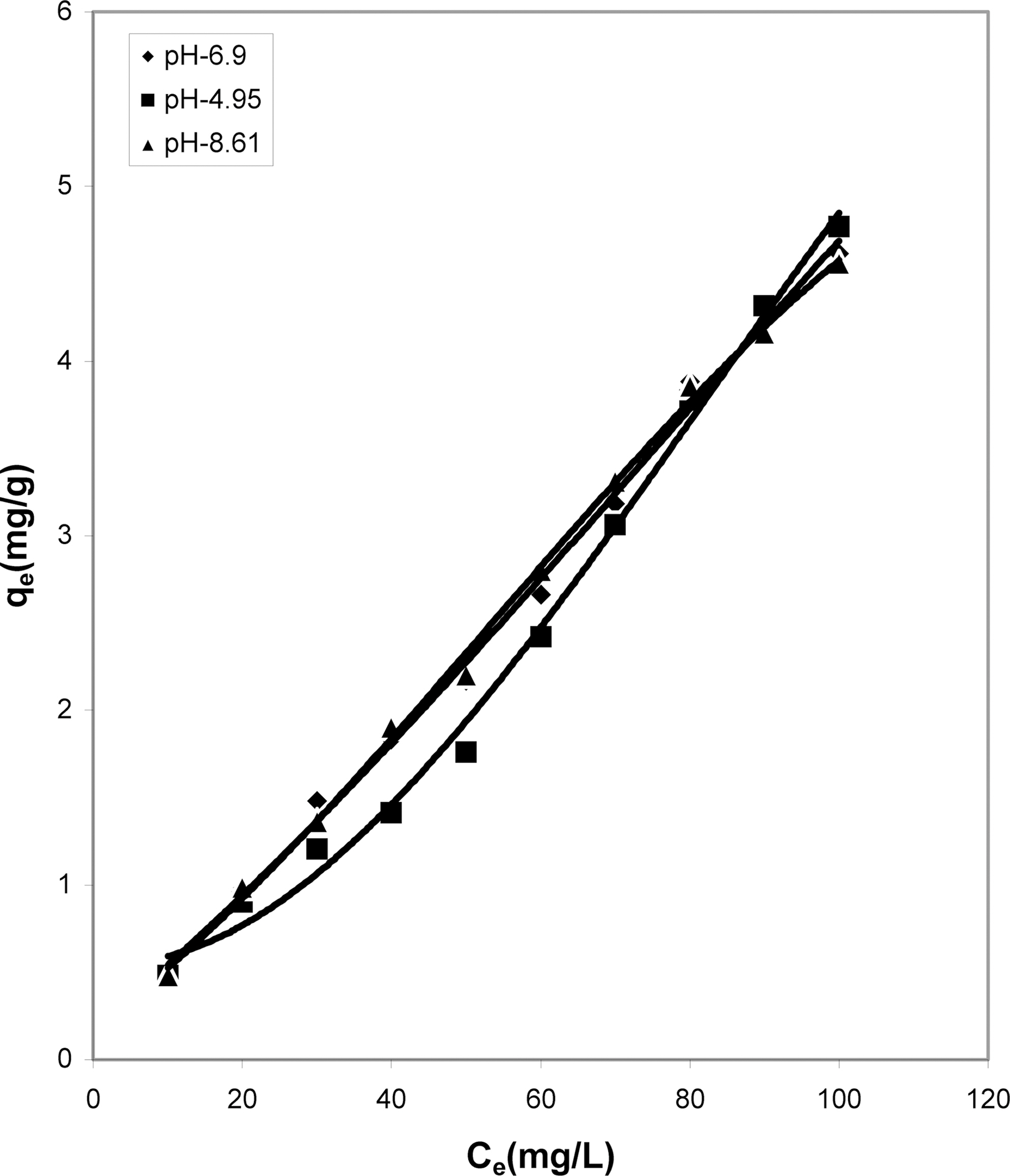
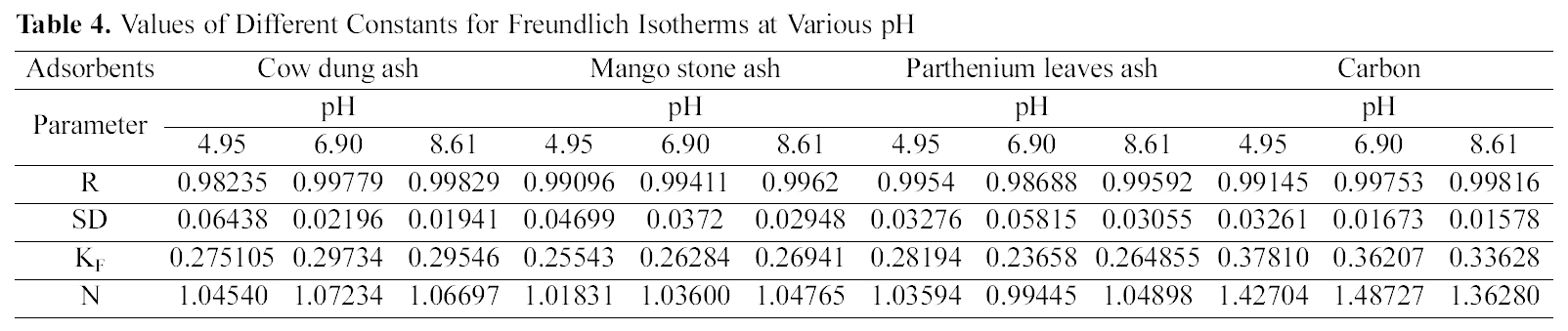
Concentration of Dye (Ce) and Amount of Dye Adsorbed Per Gram of the Adsorbent (qe) at Different pH
24 hr. with occasional shaking. The samples were then filtered and analyzed using UV-spectrophotometer.Wavelengths of different dyes were determined by λmax method. The pH values of solutions were adjusted by addition of H2SO4 and NaOH.
3.1. Effect of initial dye concentration
Table 1 indicates that the amount of dye adsorbed per gram of adsorbents increases with the increase in the dye concentration.
With increasing concentration of the dye the amount of dye adsorbed per gram of adsorbent increases and the increase varies for all the four adsorbents studied. It can be seen that cow dung ash showed better adsorption efficiency as compared to the other three adsorbents at neutral and basic pH (4.615 mg/g and 4.565 mg/g) whereas at acidic pH parthenium leaves ash exhibited maximum adsorption efficiency (4.84 mg/g).
pH is an important factor which determines the adsorption of dyes. A similar trend is observed for cow dung and parthenium leaves ash in Table 1 where amount adsorbed per gram of adsorbent is maximum at pH 4.95 (4.77 mg/g and 4.84 mg/g) and minimum at pH 8.61 (4.565 mg/g and 3.91 mg/g). Similarly, neutral pH (6.90) is least suited for mango stone ash and activated carbon (3.955 mg/g and 2.31 mg/g) though adsorption efficiency is more at basic pH(4.1 mg/g) for mango stone ash and at acidic pH (2.5 mg/g)for activated carbon.
3.3. Data fit for Simple Isotherms
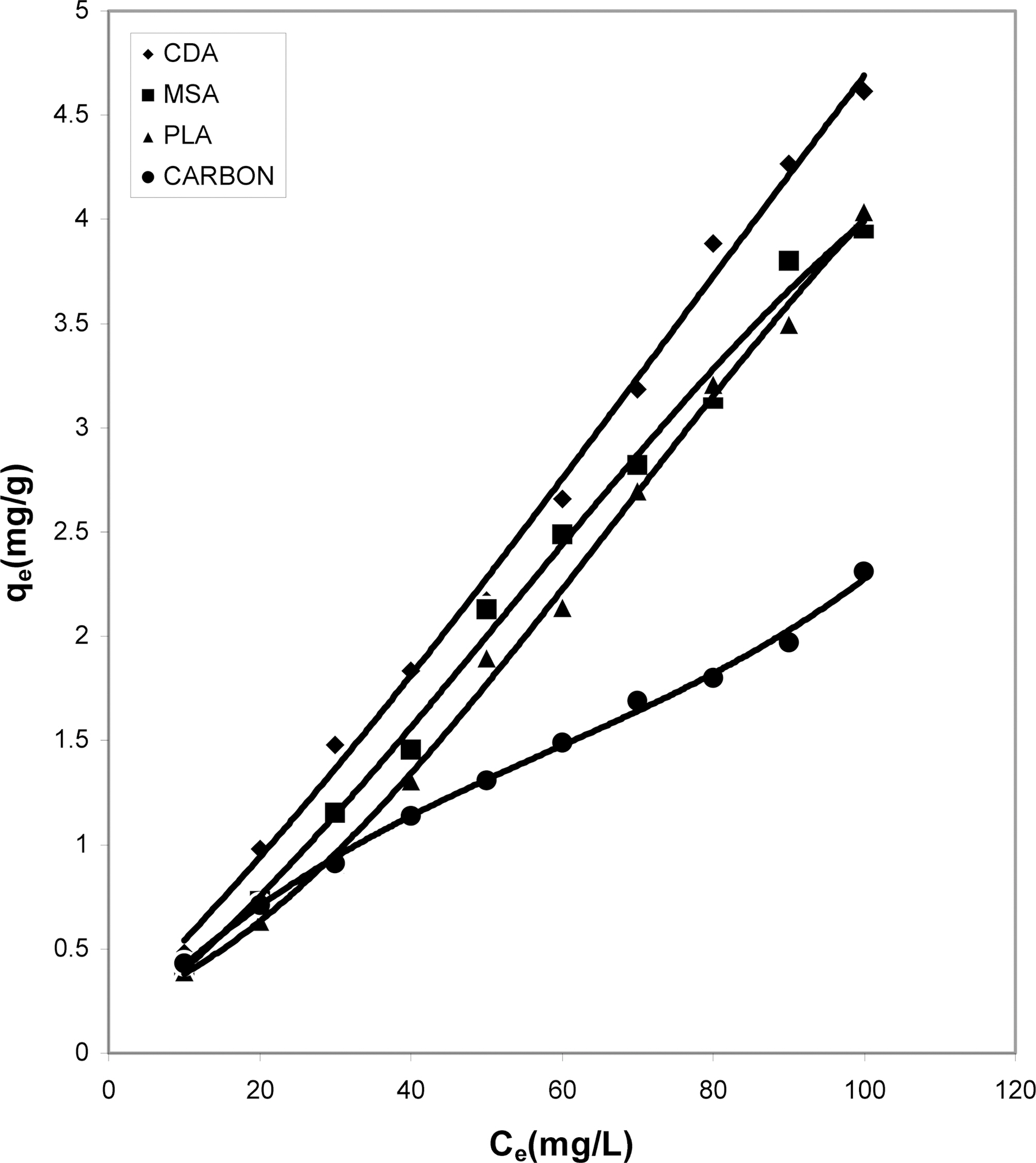
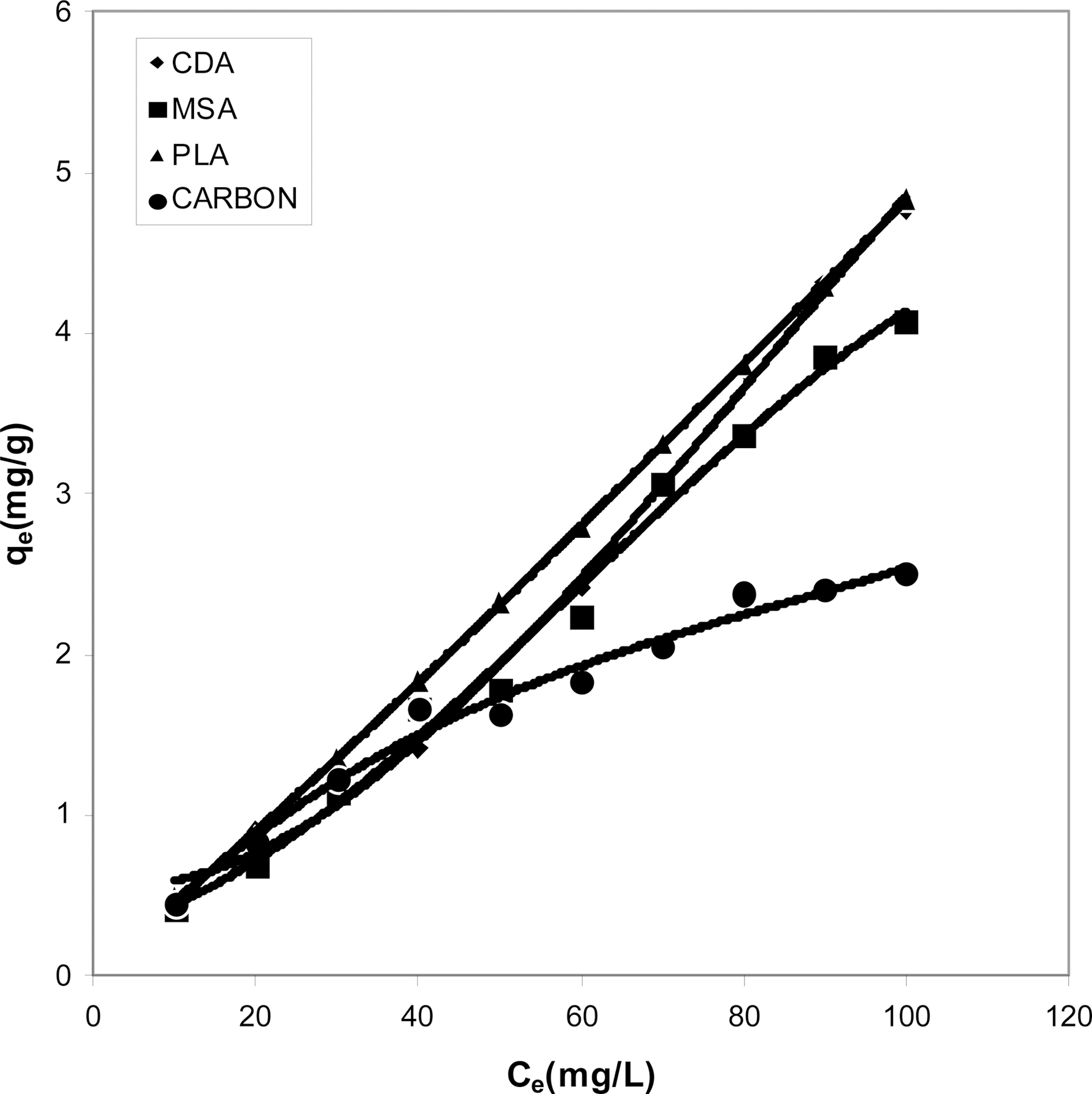
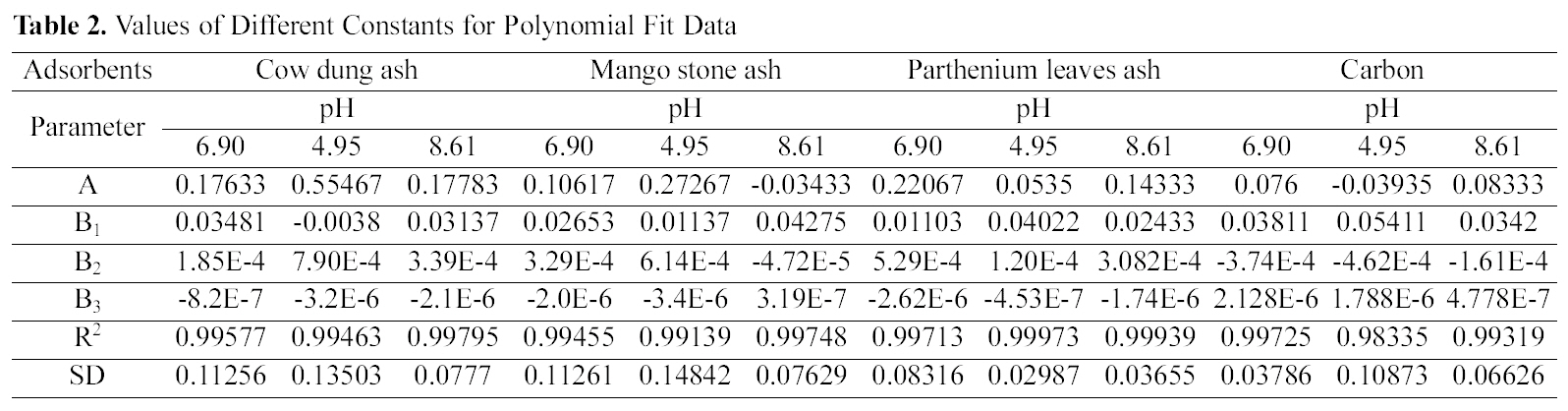
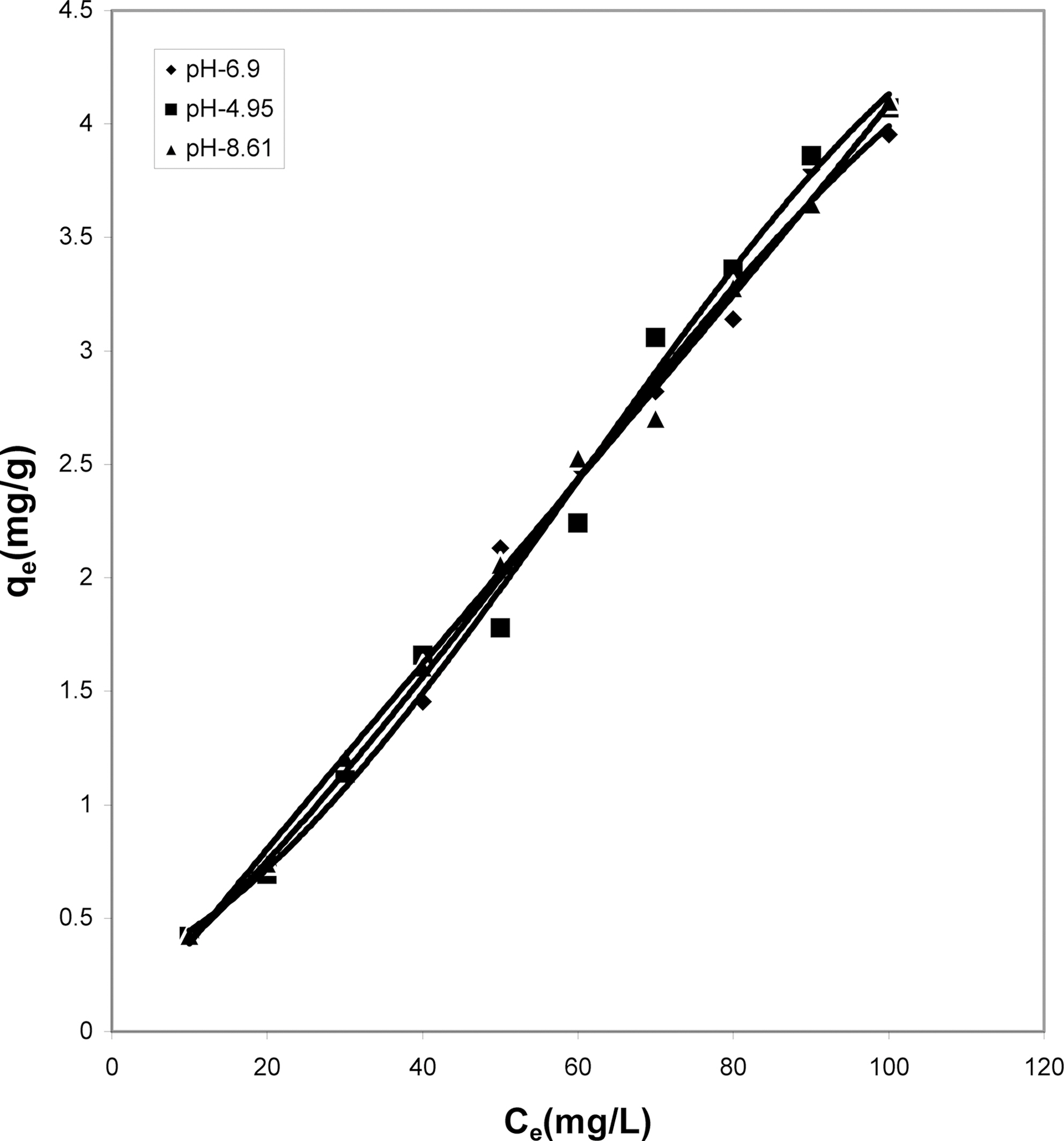
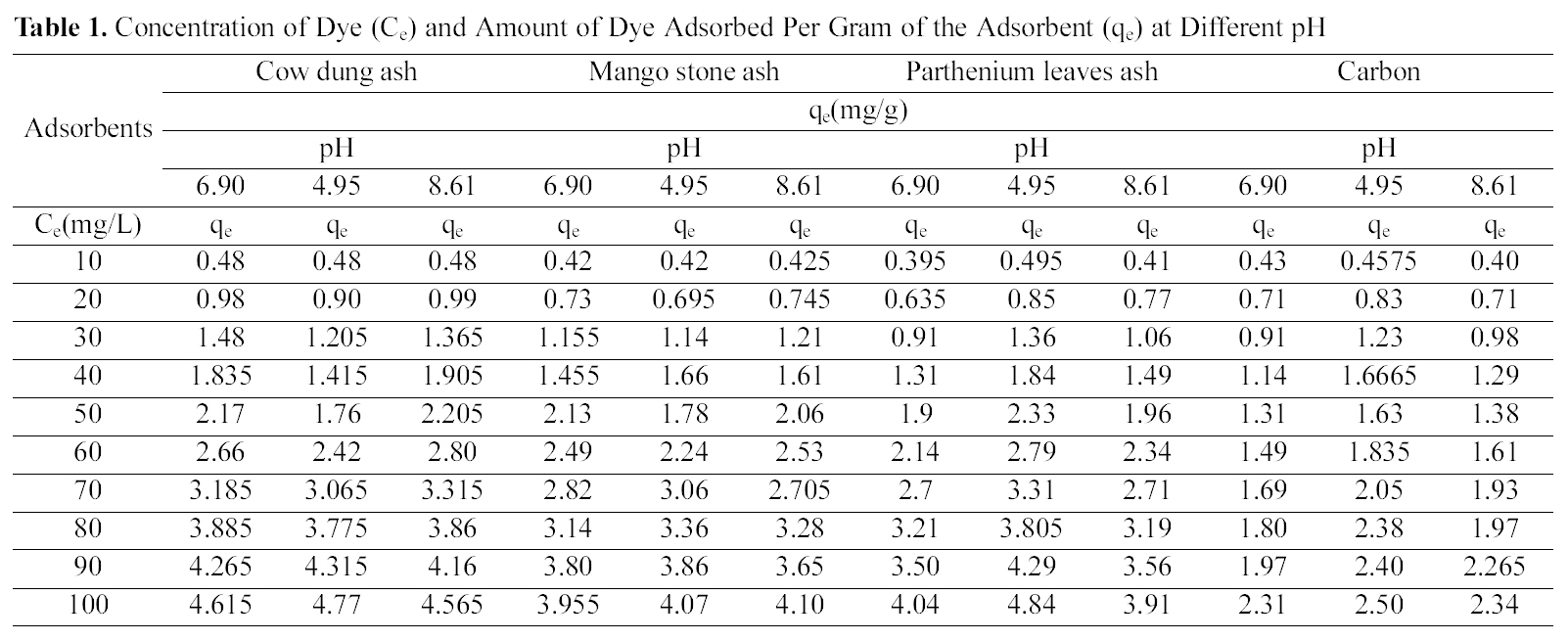
Figs. 1, 4 and 7 show that the experimental data fitted well to Polynomial Equation 1. The constants and standard deviation are given in Table 2.
Figs. 1, 4 and 7 reveal the comparative adsorption of the dye on all the four adsorbents at different pH values.Maximum removal of dye was observed at pH 4.95.Amongst all the four adsorbents activated carbon showed the
least adsorption efficiency.
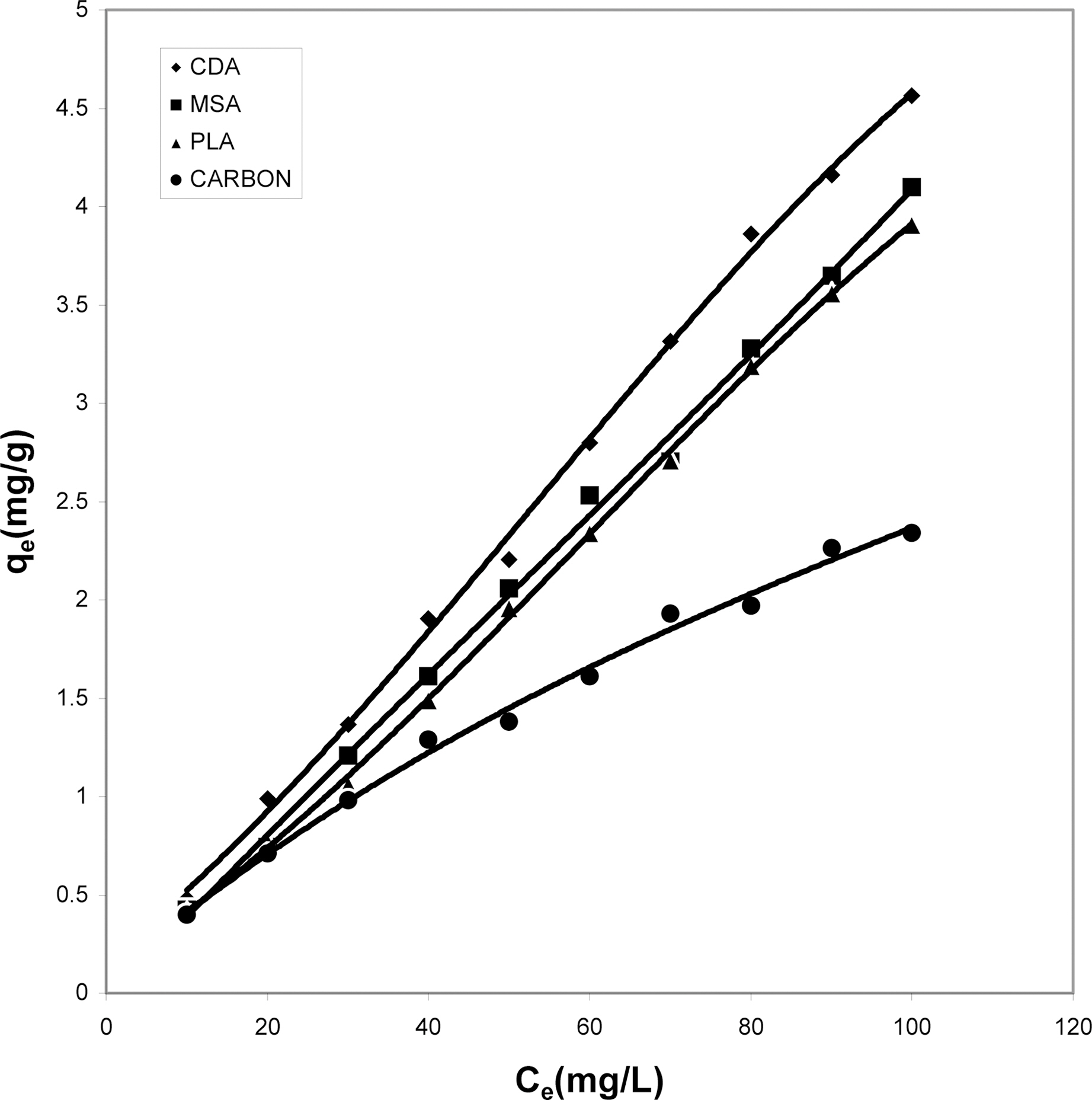
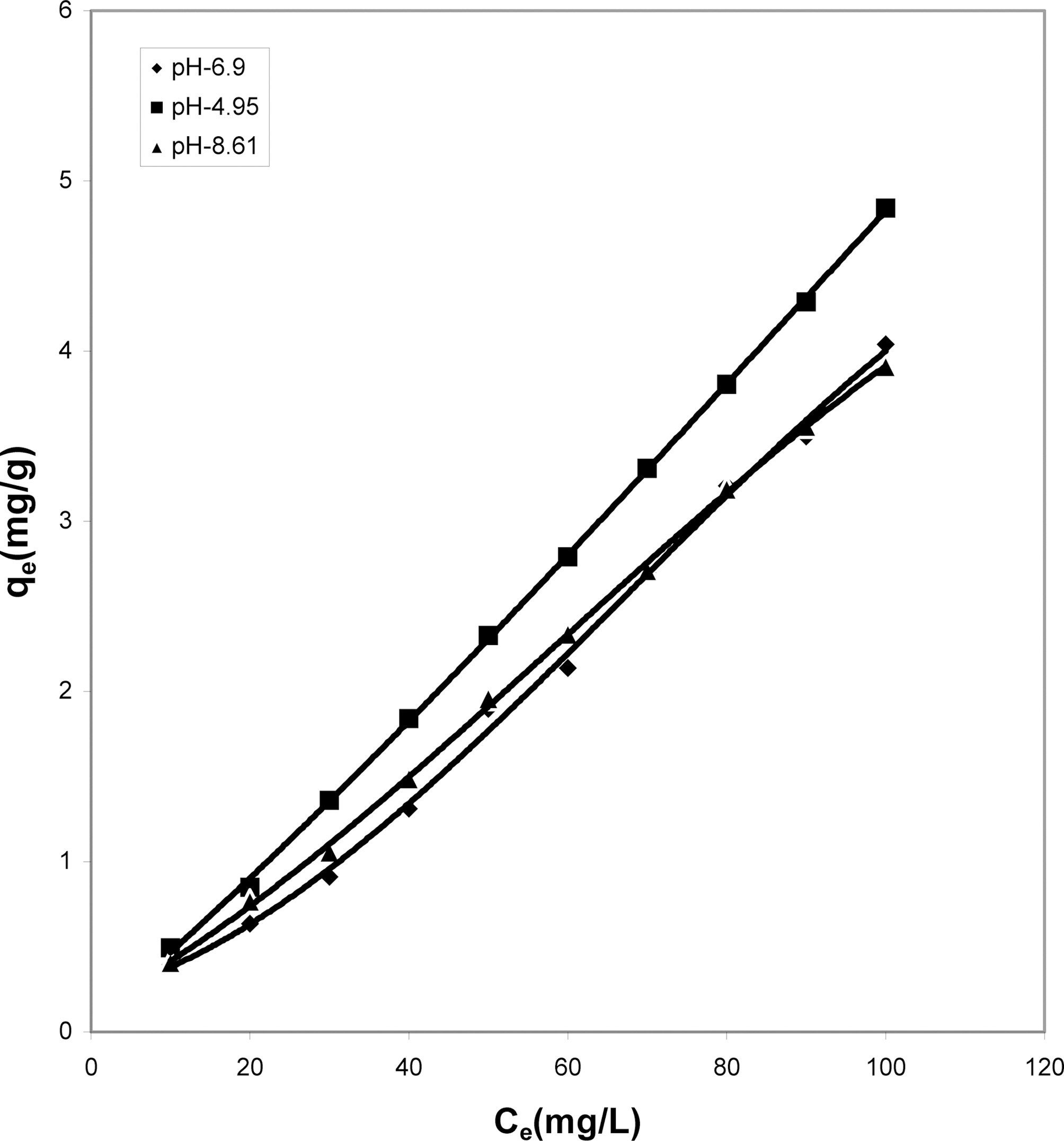
Fig. 10 shows that cow dung ash exhibits maximum color removal at higher concentration at acidic ph while at lower dye concentration the neutral and basic pH values are better.Fig. 11 clearly shows that basic pH dominates the adsorption capacity over the acidic and neutral pH.
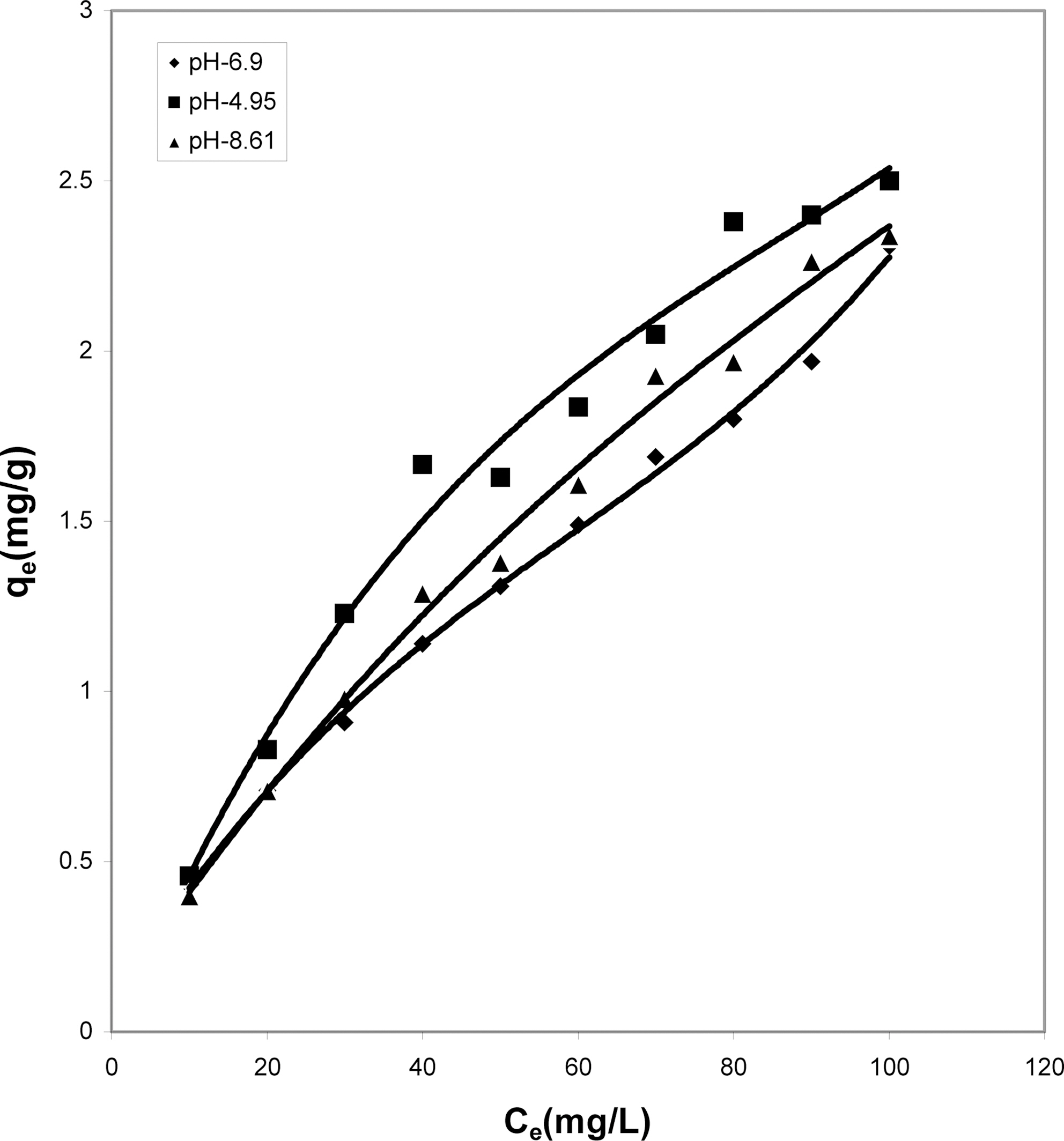
From Fig. 12, one can clearly see the capacity of
parthenium leaves ash to adsorb maximum color at the acidic pH followed by the neutral and finally basic pH. For activated carbon (Fig. 13) it can be drawn that simple isotherm shows the same behavior for all three pH values i.e.with increase in concentration the adsorption of dye increases. Both acidic and basic pH values are better suited
[Table 2.] Values of Different Constants for Polynomial Fit Data
Values of Different Constants for Polynomial Fit Data
than the neutral condition. But acidic solution favors better adsorption from other two pH values.
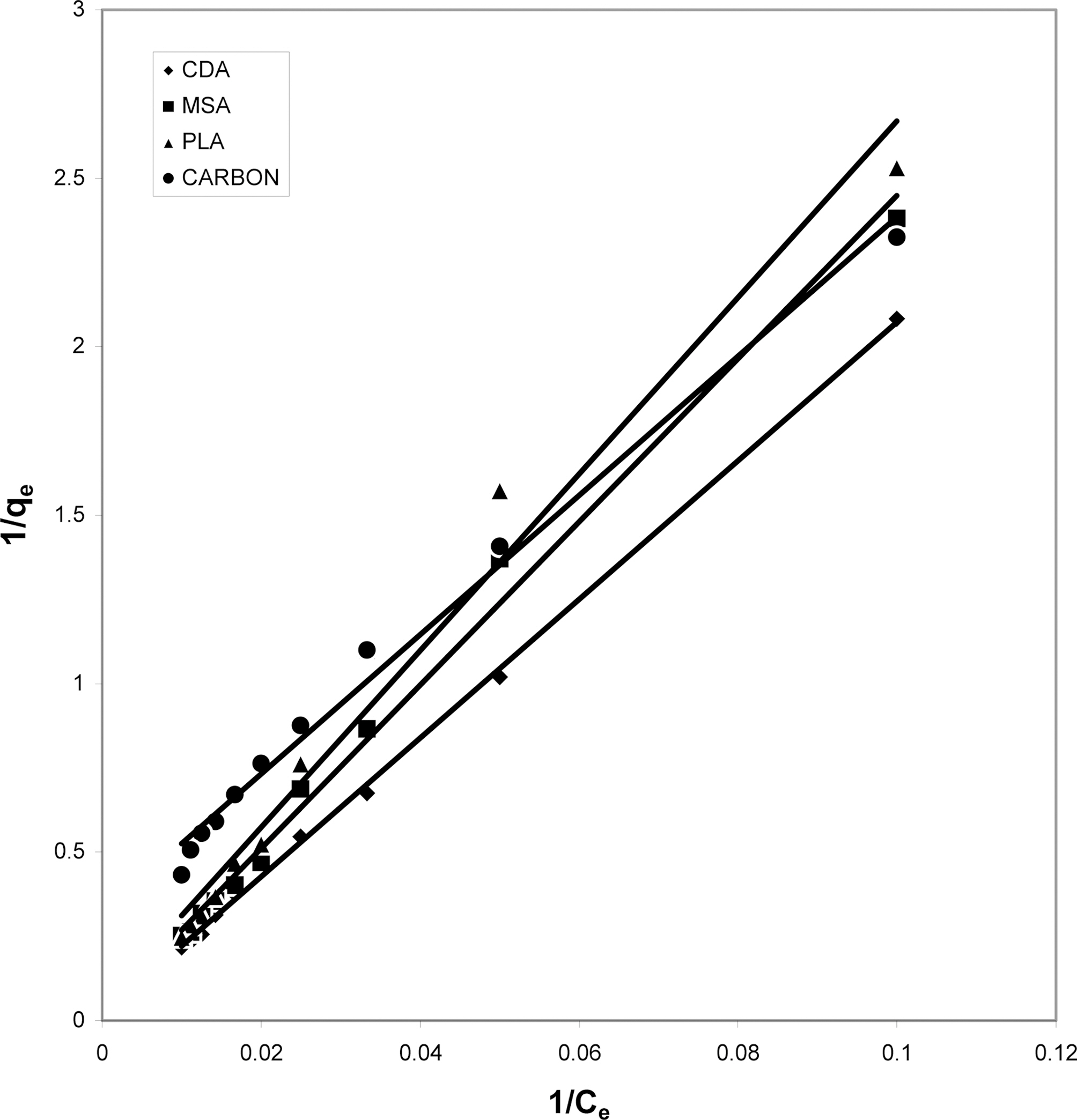
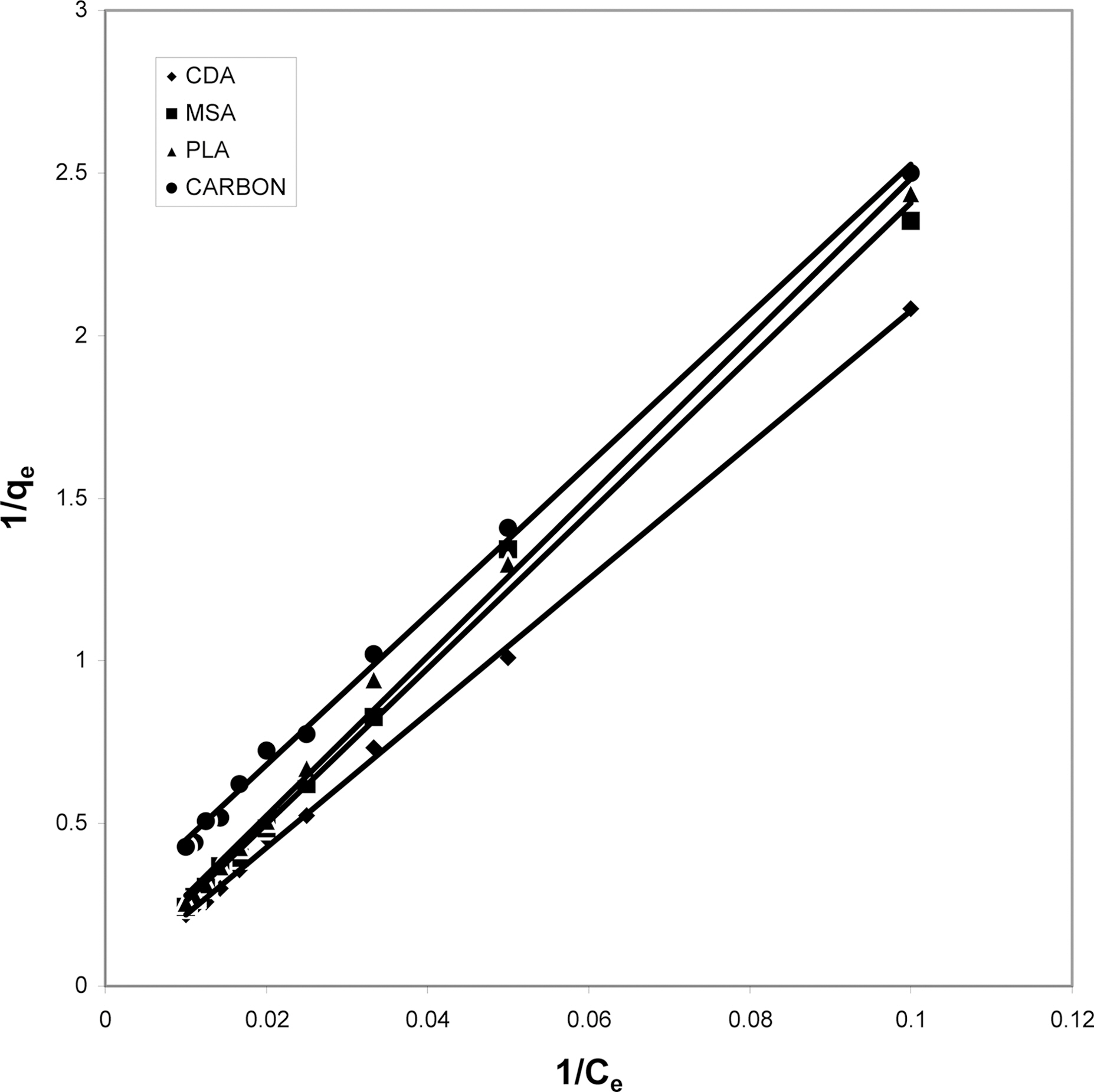
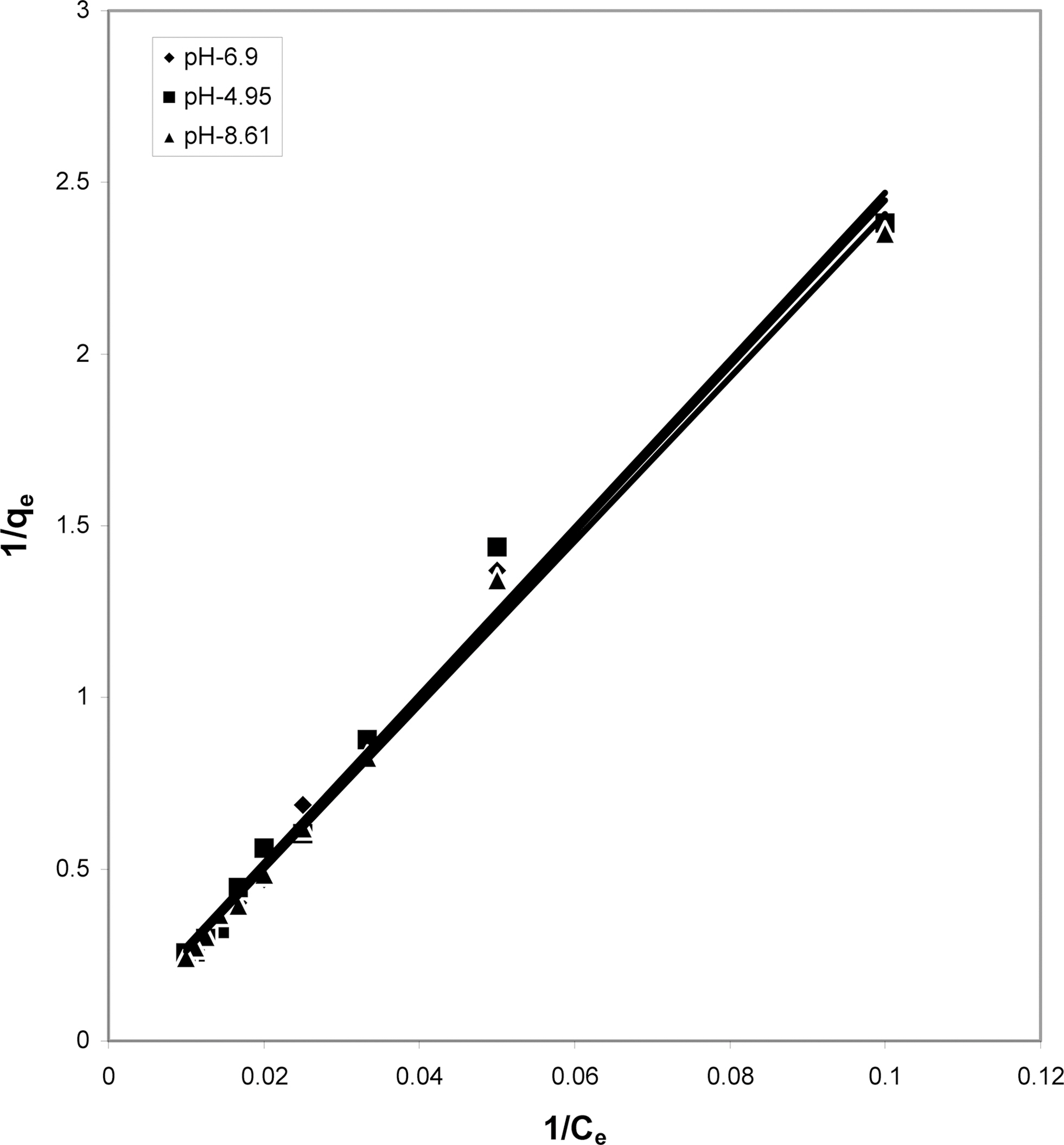
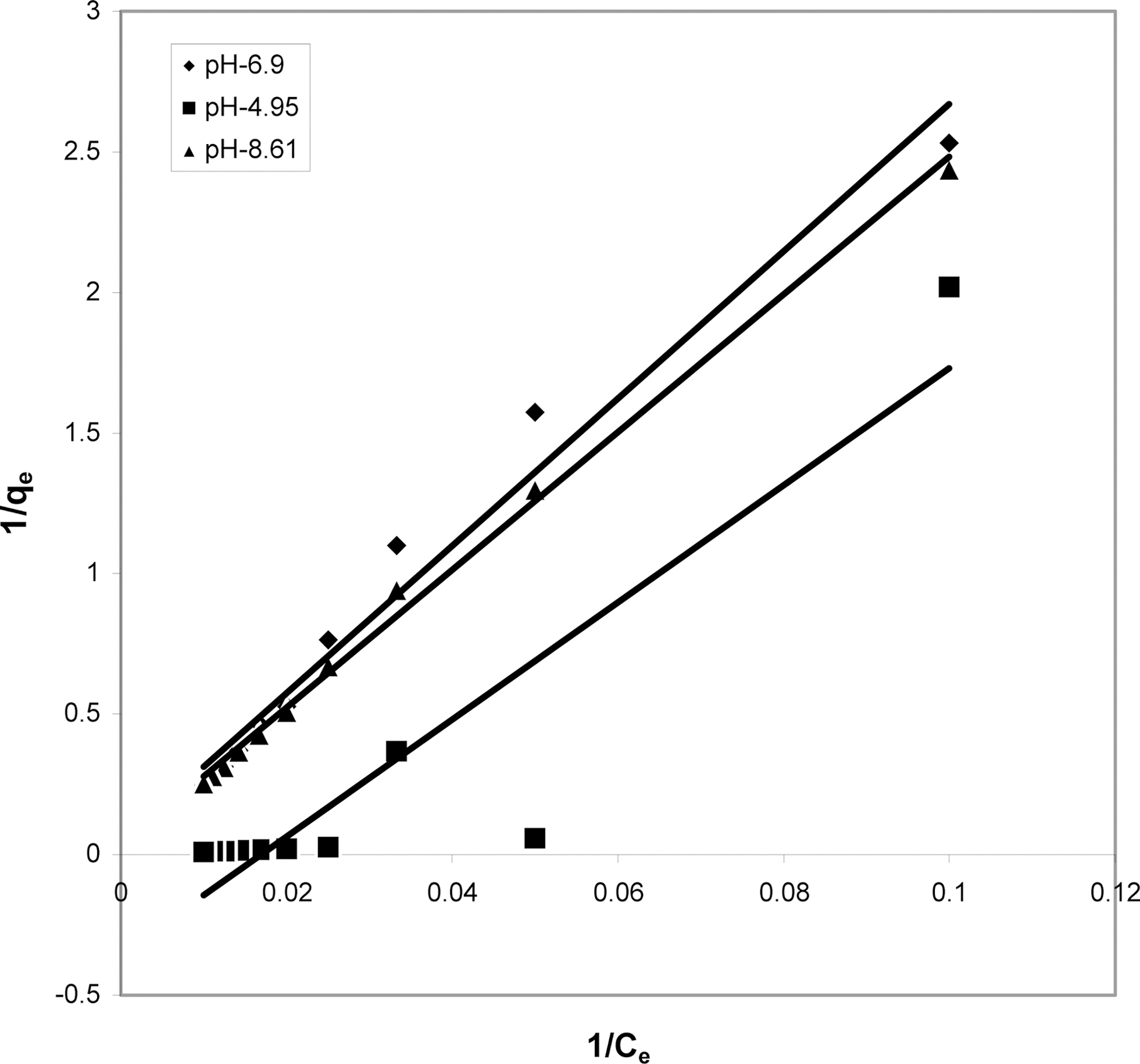
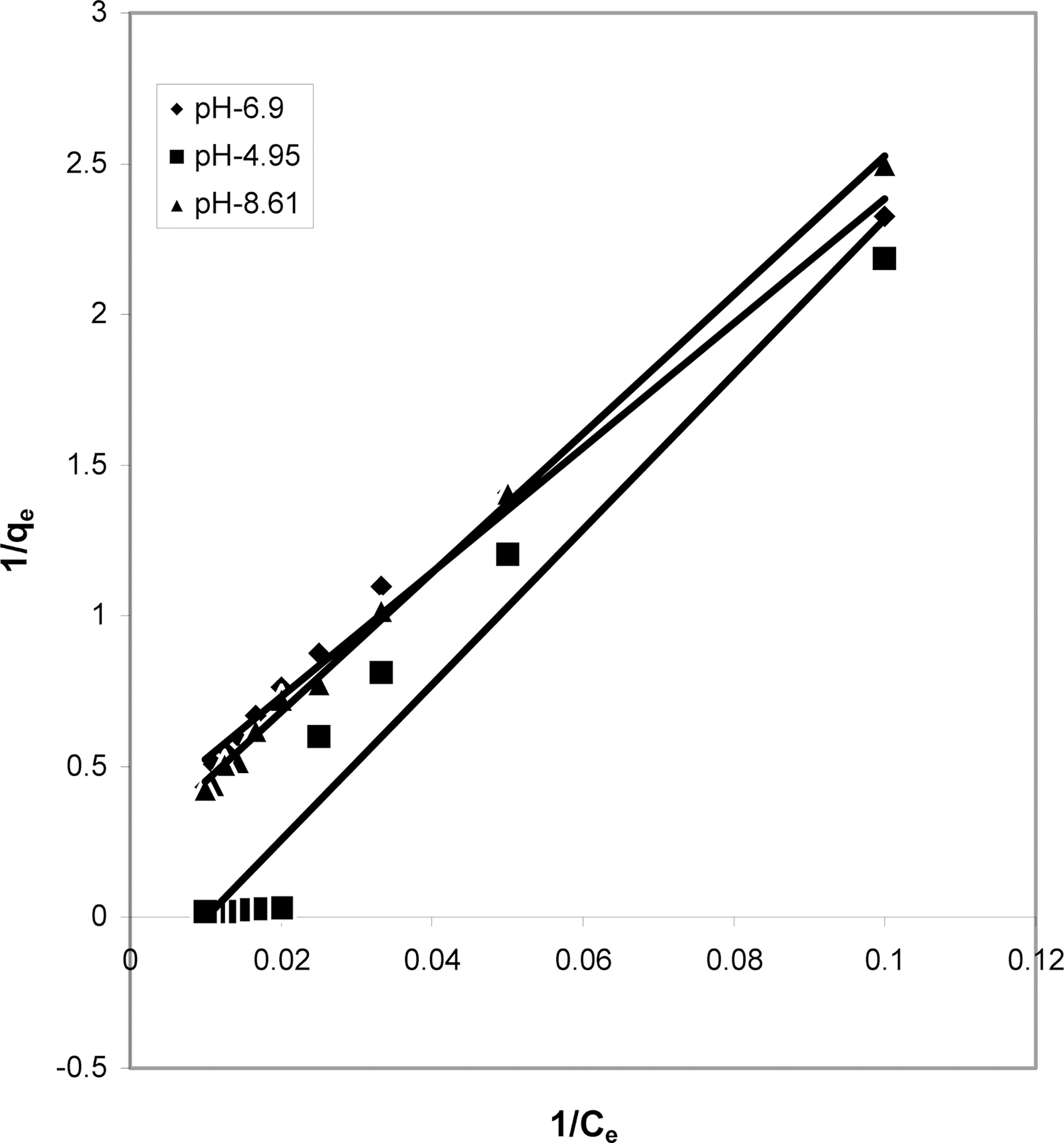
3.4. Langmuir isotherms at various pH
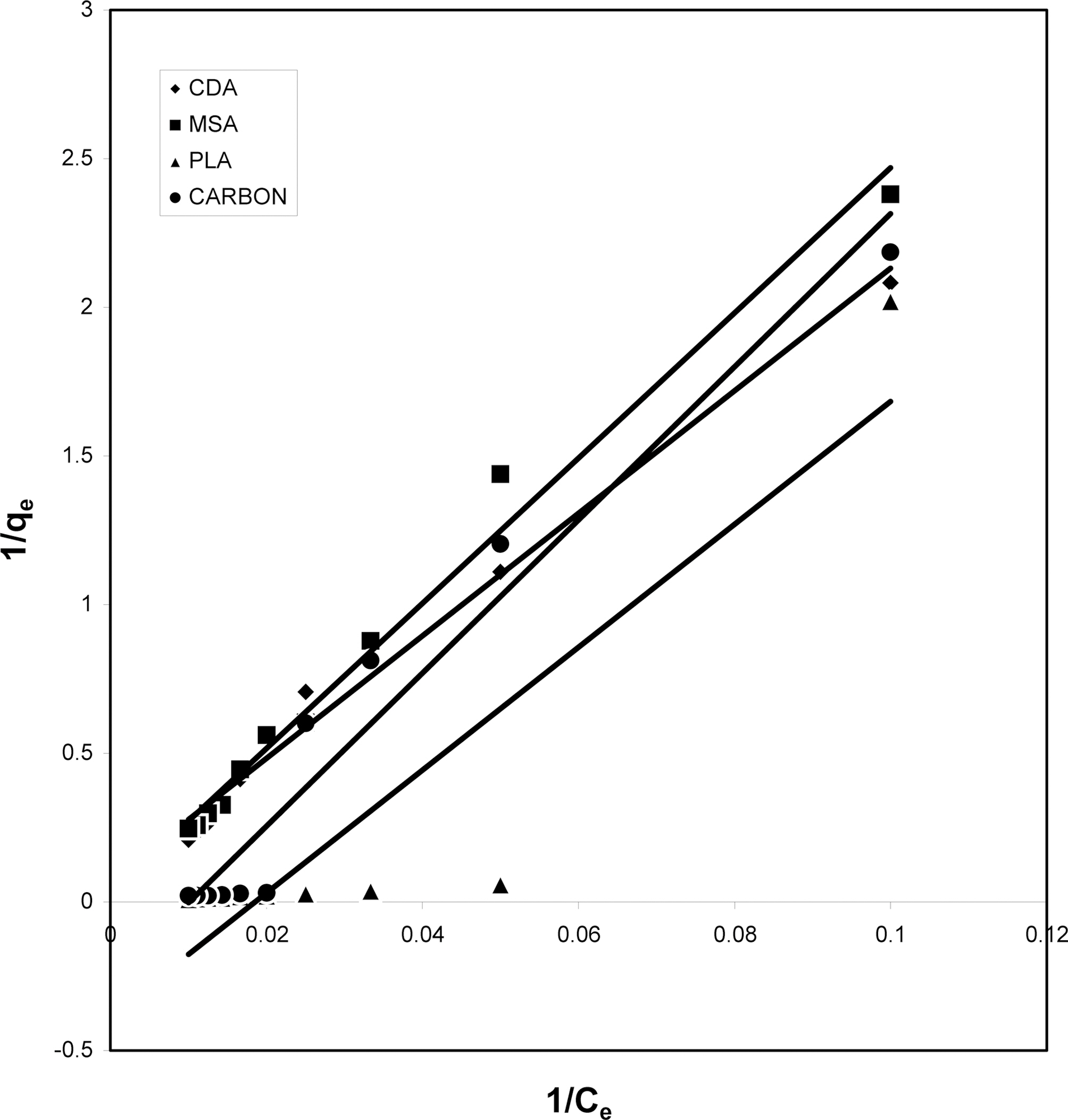
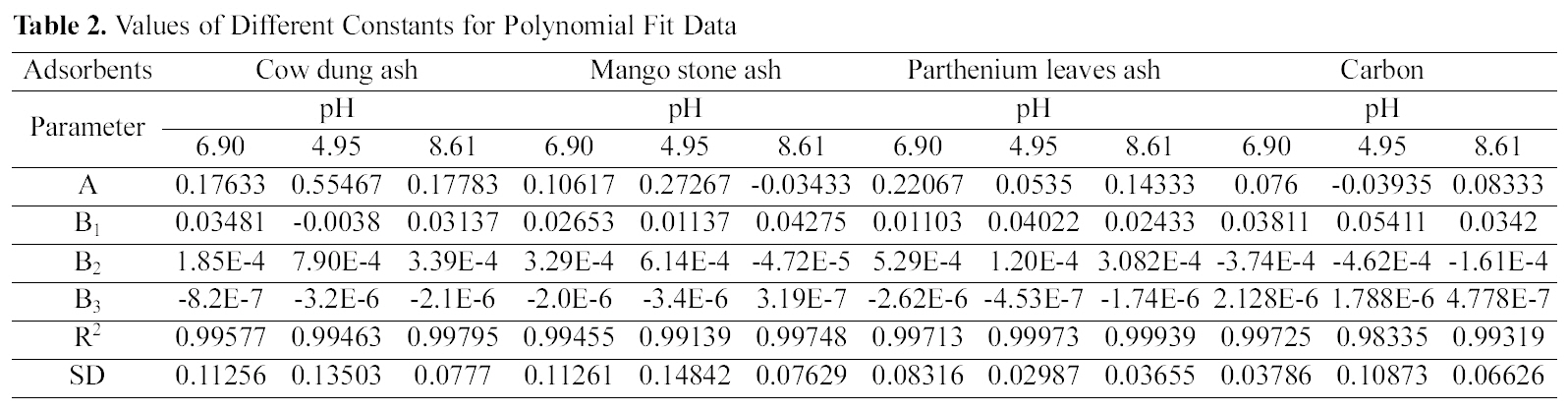
The data obtained was fitted to Langmuir isotherm as
shown in Figs. 2, 5 and 8 and also Figs. 14~17 for all the four adsorbents at three different pH values.
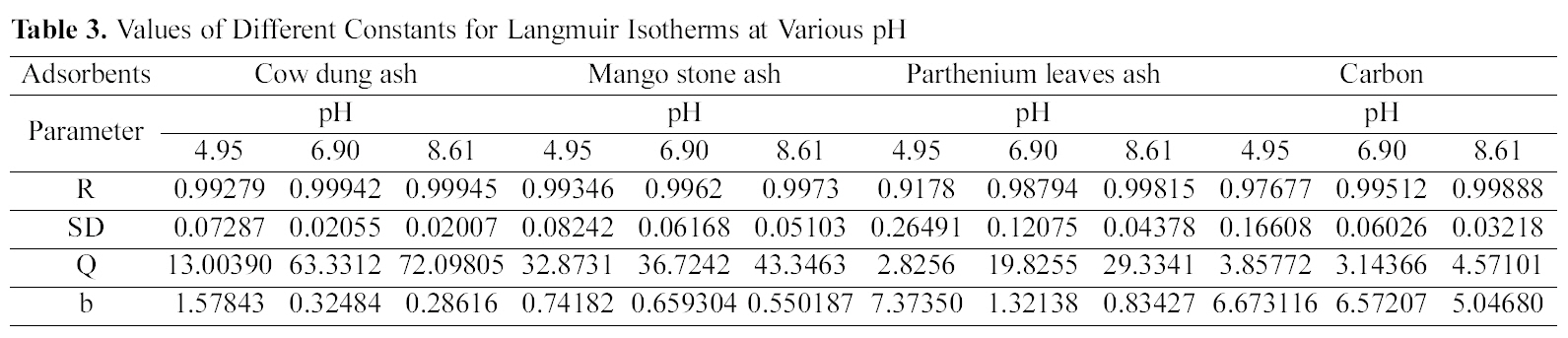
Table 3 gives the values of Langmuir constants “Q”
(adsorption capacity in mg/g) and “b” (energy of adsorption in mg/L) along with the dimensionless separation factor R and standard deviation SD.
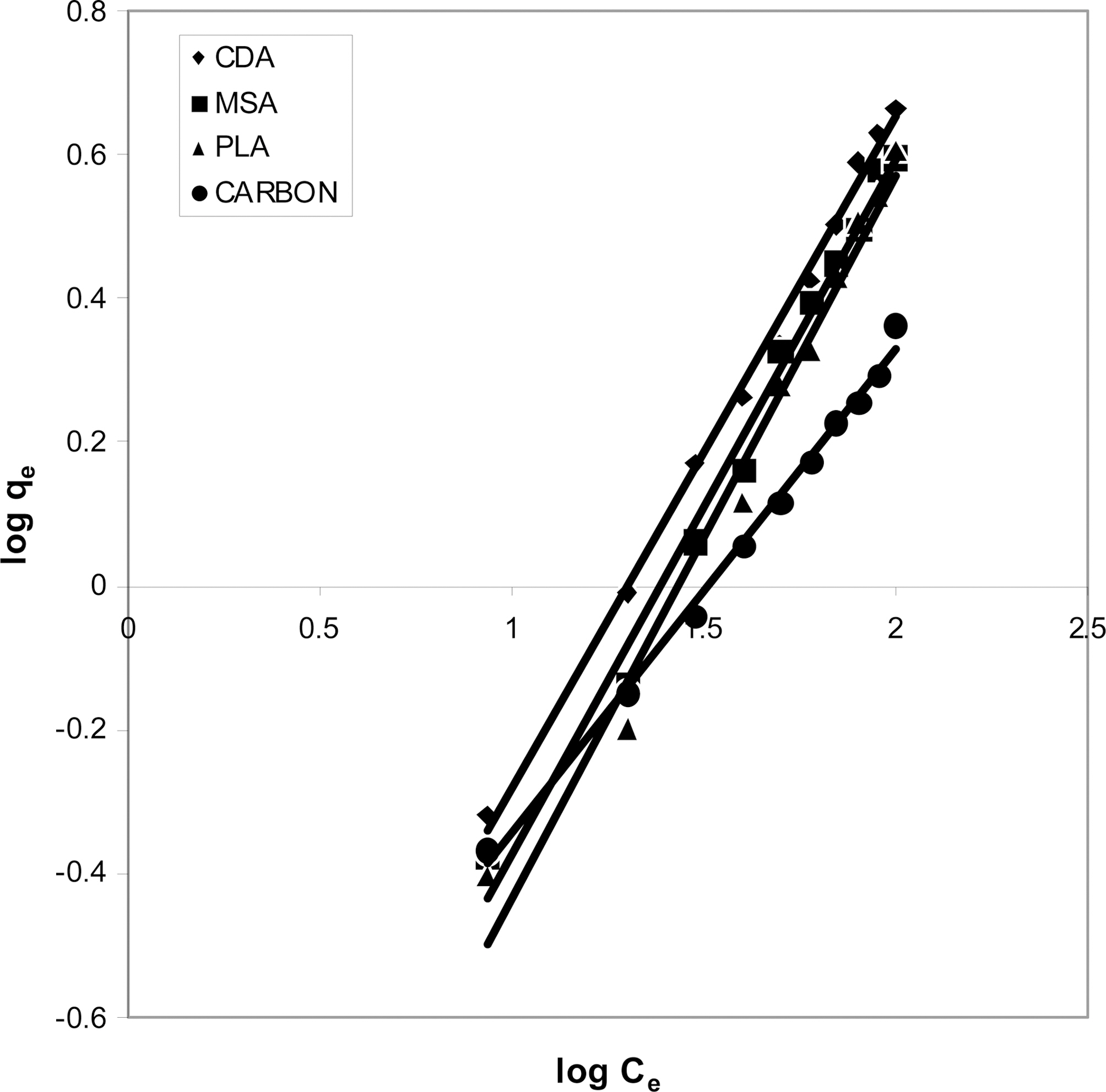
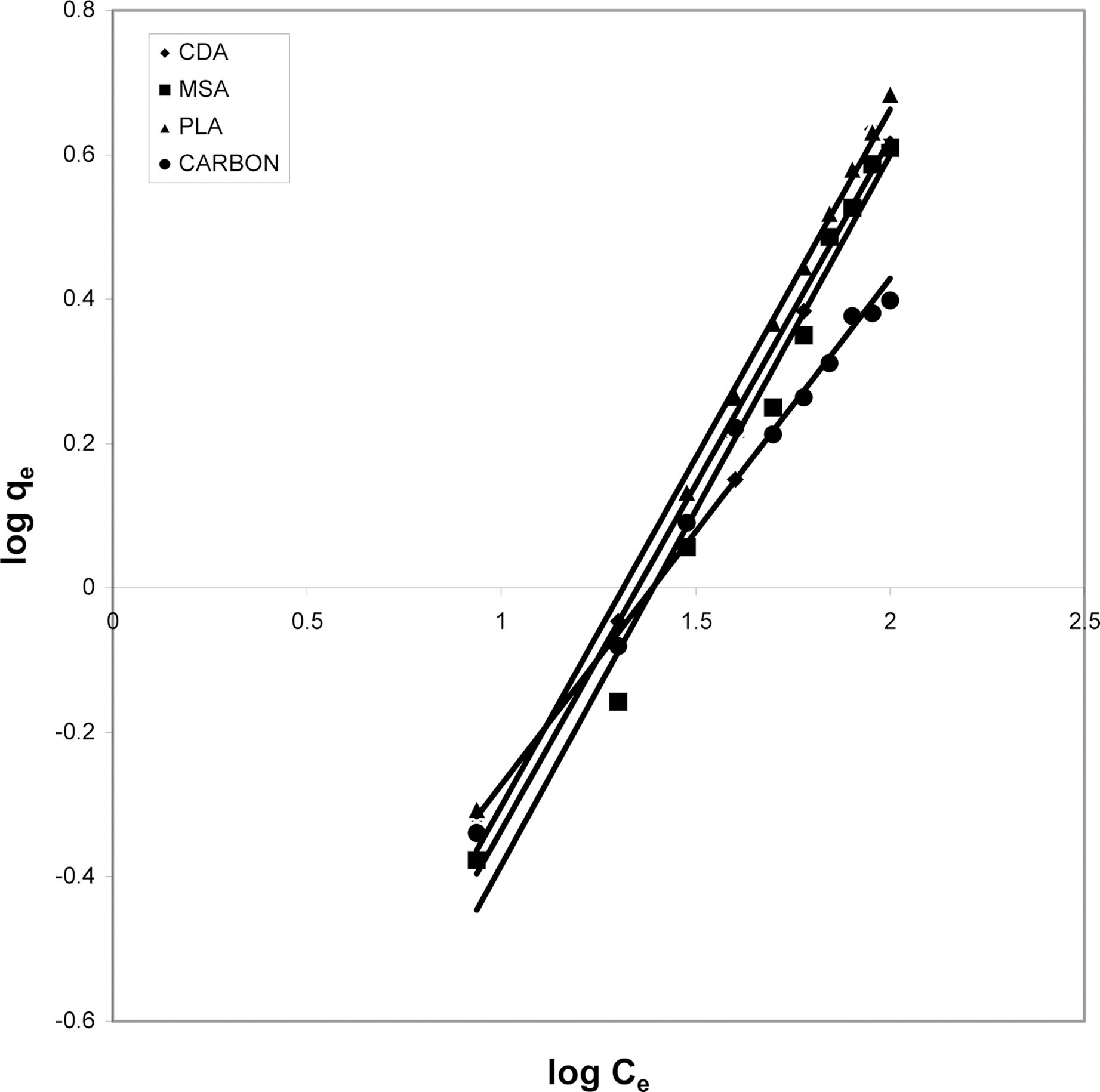
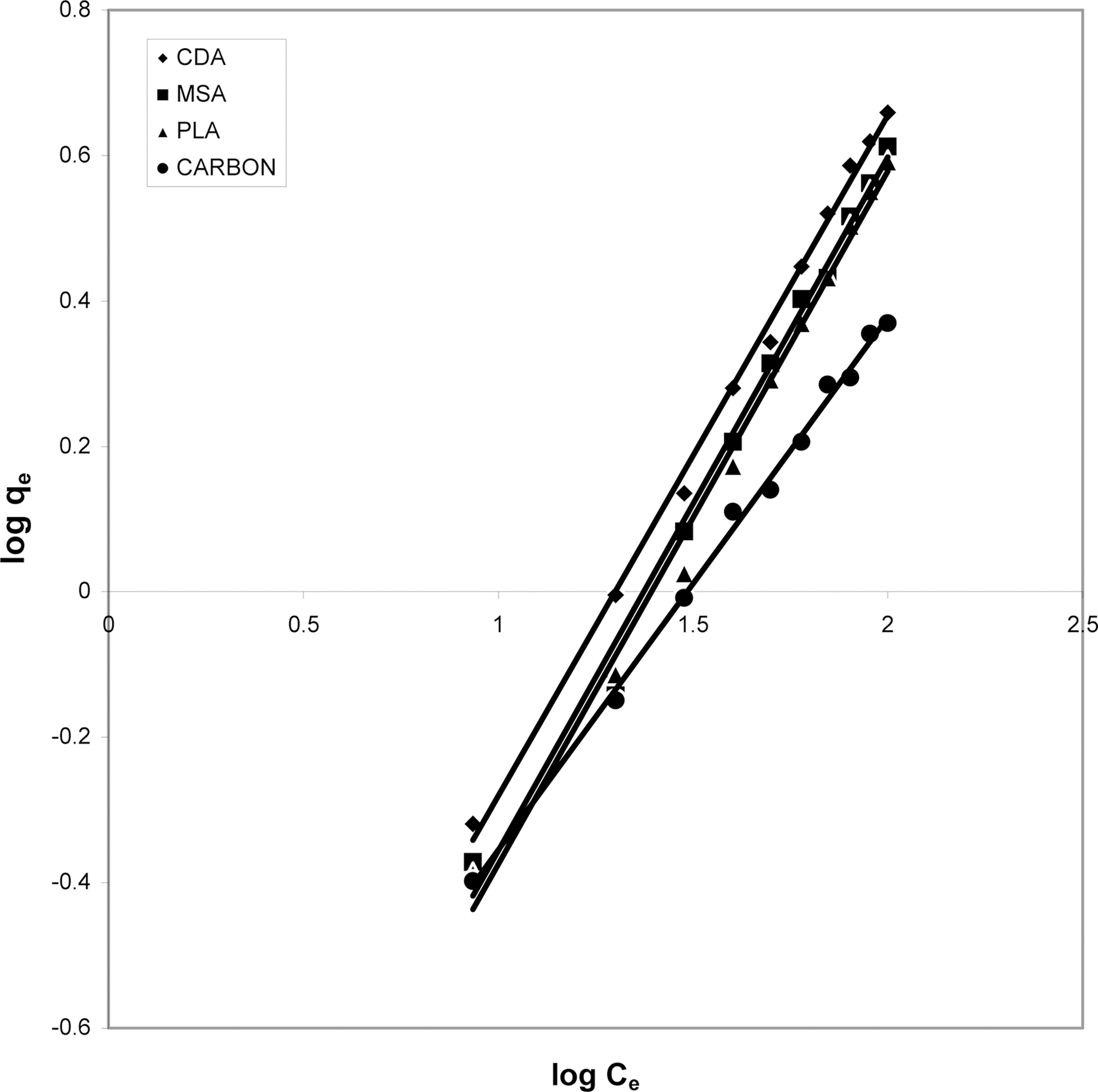
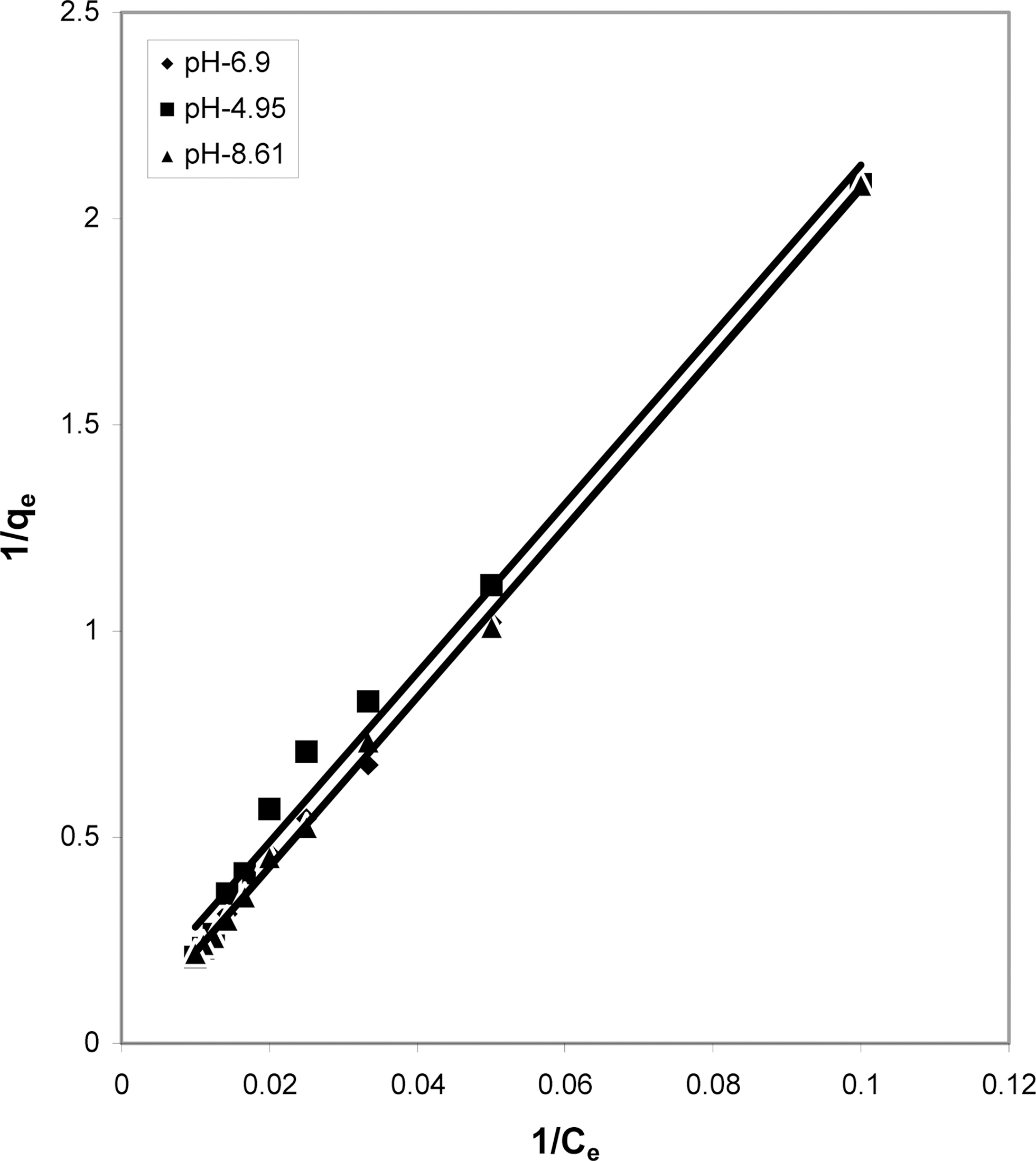
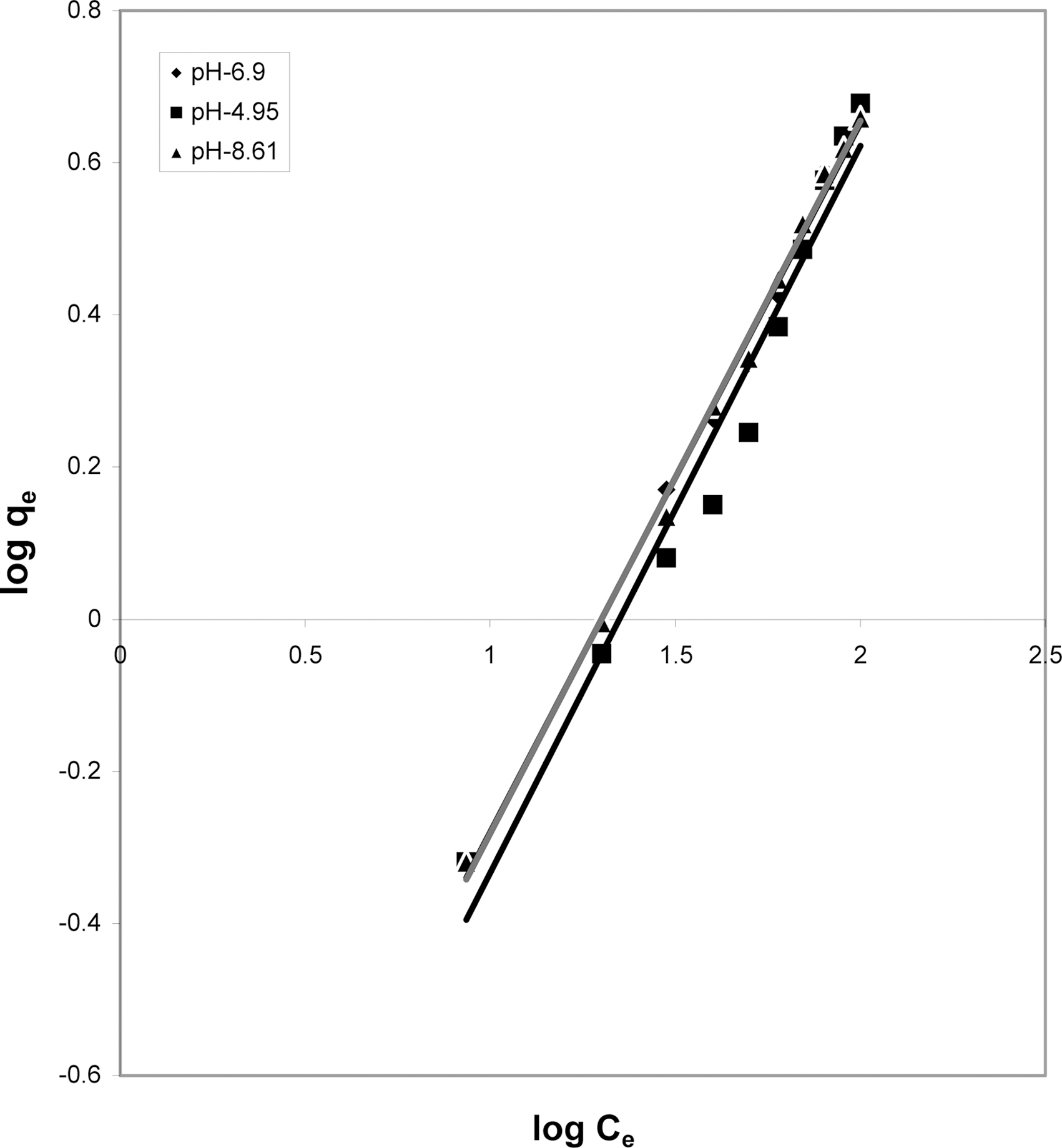
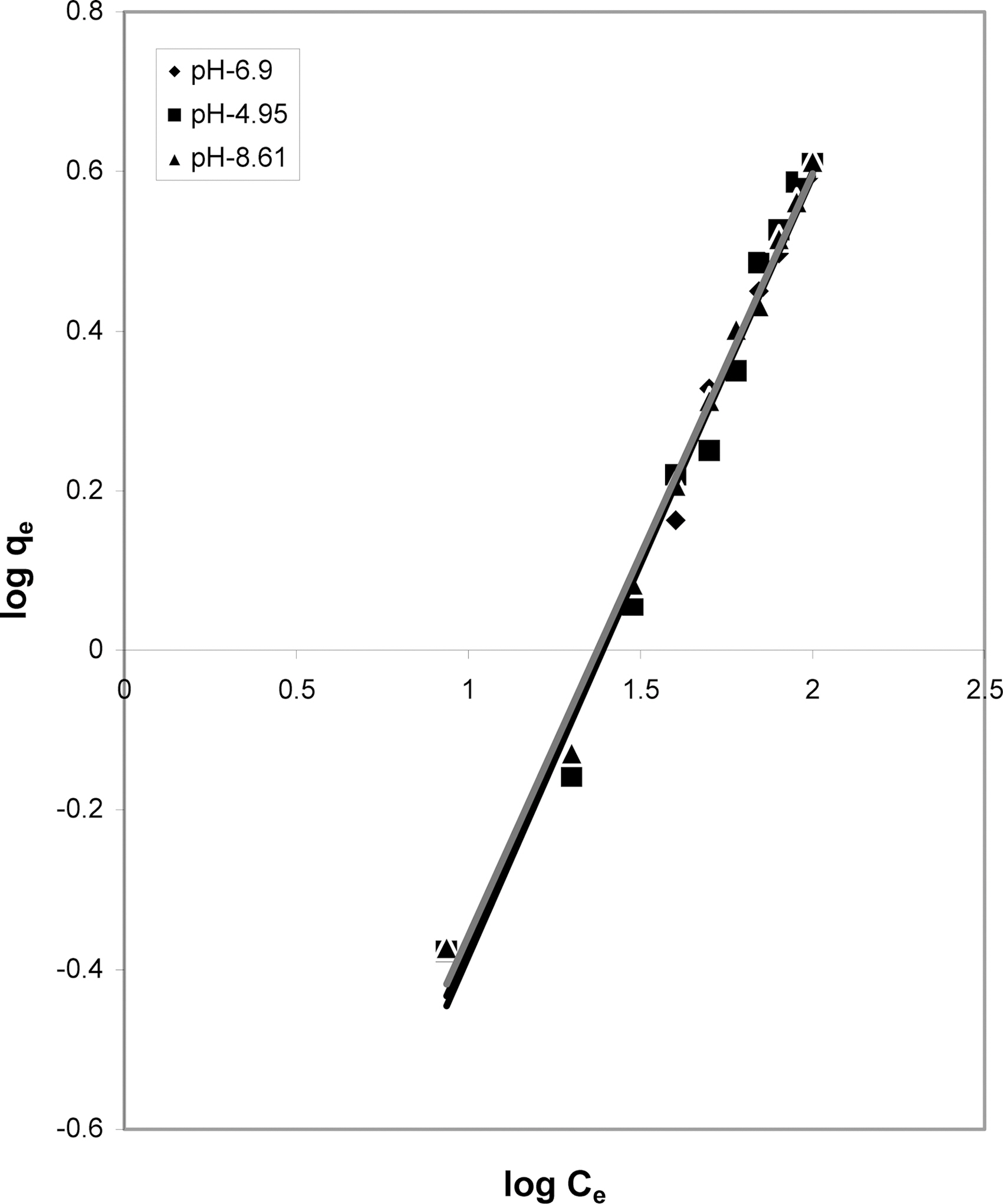
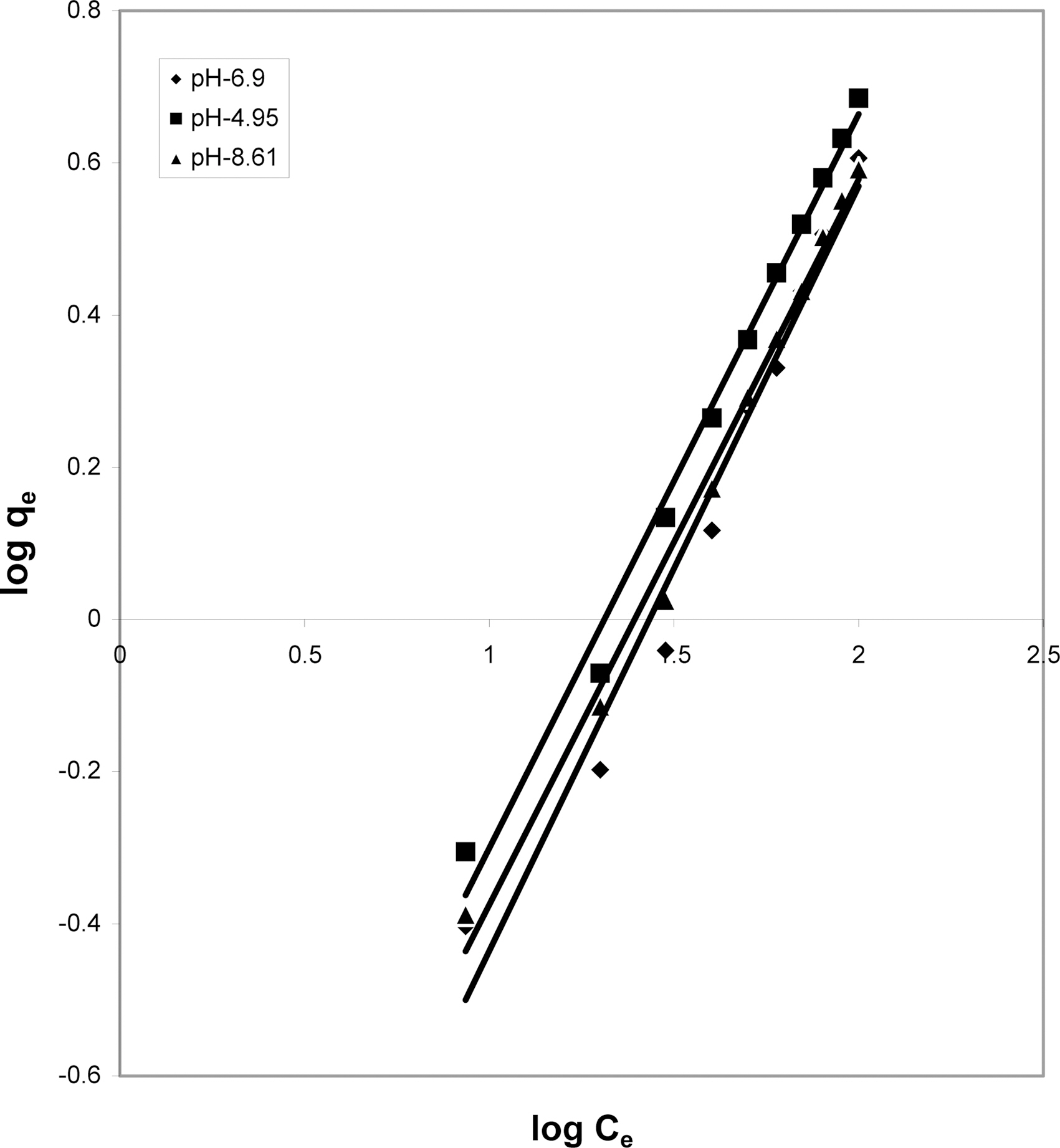
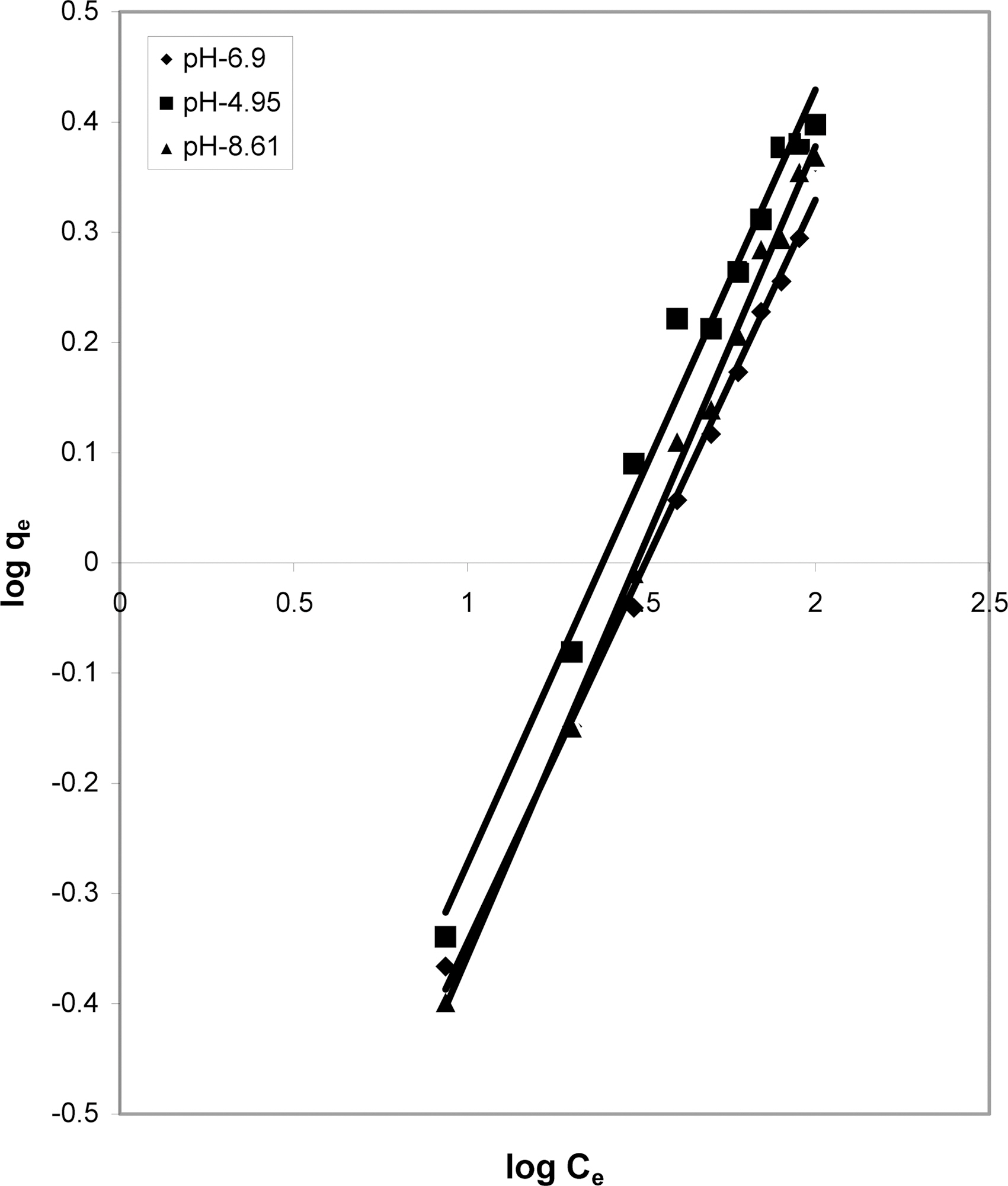
3.5. Freundlich isotherm at various pH
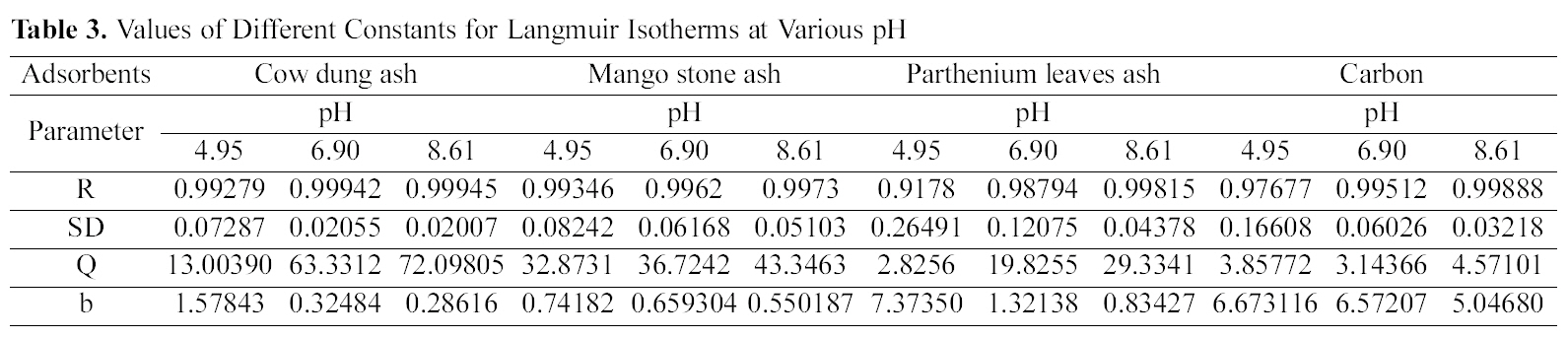
The data was also fitted to the Freundlich isotherm as
[Table 3.] Values of Different Constants for Langmuir Isotherms at Various pH
Values of Different Constants for Langmuir Isotherms at Various pH
[Table 4.] Values of Different Constants for Freundlich Isotherms at Various pH
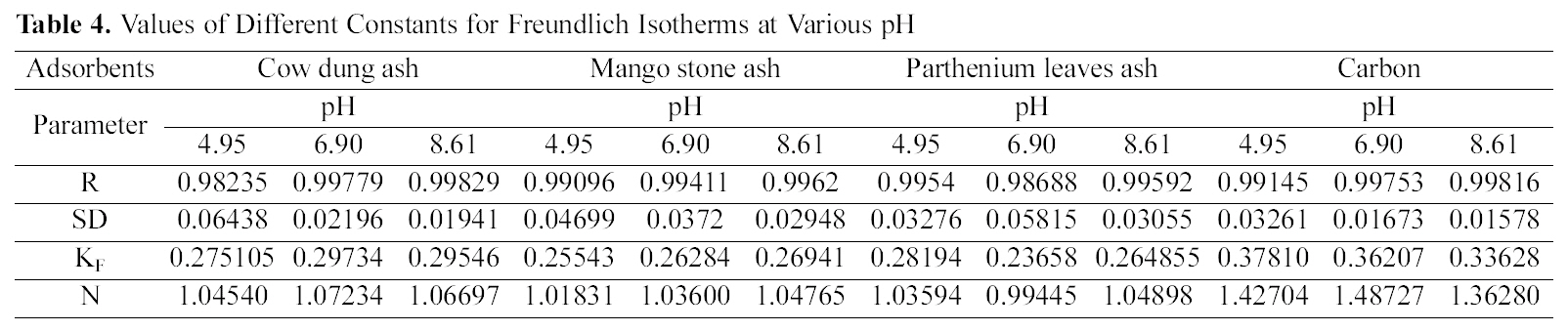
Values of Different Constants for Freundlich Isotherms at Various pH
shown in Figs. 3, 6 and 9 and also Figs. 18~21 at various pH values. Freundlich constants “KF” (adsorption capacity) and“n” (process intensity) have also been calculated from the slope and intercept of log qe and log Ce and their values along with R and standard deviation SD are given in Table 4.
The results obtained from the present investigation reveal the ability of various biomass ashes in treating dye effluents released from the tanneries for color removal. Adsorption of dye studied on various biomass ash and activated carbon shows that with increase in concentration of dye there is decrease in percentage removal of the dye per gram.Adsorption is highly dependent on pH and dye concentration. The adsorption isotherm of Acid Blue 92 onto the cow dung ash, mango stone ash, parthenium leaves ash and activated carbon is described by the Langmuir and Freundlich isotherm models. Judging by the value of R Langmuir isotherm gave a better fit for cow dung and mango stone ash whereas for parthenium leaves ash and activated carbon both the isotherms fitted equally well.The different values of Q are explained by the varying degree of interaction between the adsorbate and the adsorbent. These agriculture waste residues could therefore be substituted in place of activated carbon as adsorbent due to its availability,high adsorption capacity and low cost.