Biogenic Mn oxides are expected to have great potential in the control of water pollution due to their high catalytic activity, although information on biological Mn oxidation is not currently sufficient. In this study, the growth of a Mn oxidizing microorganism, Pseudomonas putida MnB1, was examined, with the Mn oxides formed by this strain characterized. The growth of P. putida MnB1 was not significantly influenced by Mn(II), but showed a slightly decreased growth rate in the presence of Pb(II) and EE2, indicating their insignificant adsorption onto the cell surface. Mn oxides were formed by P. putida MnB1, but the liquid growth medium and resulting biogenic solids were poorly crystalline, nano-sized particles. Biogenic Mn oxidation by P. putida MnB1 followed Michaelis-Menten kinetics, with stoichiometric amounts of Mn oxides formed, which corresponded with the initial Mn(II) concentration. However, the formation of Mn oxides was inhibited at high initial Mn(II) concentration, suggesting mass transfer obstruction of Mn(II) due to the accumulation of Mn oxides on the extracellular layer. Mn oxidation by P. putida MnB1 was very sensitive to pH and temperature, showing sharp decreases in the Mn oxidation rates outside of the optimum ranges, i.e. pH 7.43-8.22 and around 20-26℃.
Manganese oxides (Mn oxides) promote the oxidation of a variety of synthetic and natural organic pollutants, such as humic substances, phenols, endocrine disrupters and chlorinated organic compounds, due to their high redox potential [1-4]. Recently, biologically generated Mn oxides have been of increasing interest due to their high available surface area [5]. Microorganisms (bacteria and fungi) that oxidize Mn(II) to Mn(IV) oxides are known as Mn-oxidizing microorganisms. The Mn-oxidizing bacteria present in raw water can grow and reproduce under appropriate conditions and are able to oxidize Mn(II), with precipitation of the oxidized form, Mn(IV). The biogenic Mn oxides formed by Mn-oxidizing bacteria have many specific characteristics, such as high specific surface areas and reactivities, and can be used for adsorbents, catalysts, and oxidants or reductants [6]. Under circumneutral conditions, the microbial oxidation of Mn(II) proceeds several orders of magnitude faster than the abiotic oxidation, such as in homogenous and mineral surfacecatalytic reactions [5]. Therefore, the microbial Mn(II) oxidation process has gained increasing attention with regard to its environmental applications.
Studies on biogenic Mn oxidation have focused on several organisms, including
The objectives of this study were to characterize the growth of
2.2. Growth of Pseudomonas putida strain MnB1
Batch experiments were conducted in 300 mL Erlenmeyer culture flasks (Duran) with the liquid growth medium. For each batch experiment, one tube of
[Table 1.] Composition of #279 Broth (ATCCTM)
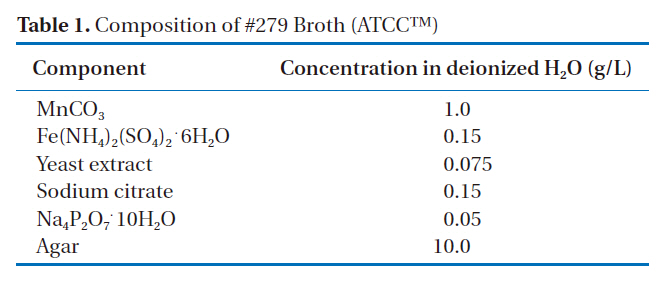
Composition of #279 Broth (ATCCTM)
[Table 2.] Compositions of Nutrient Broth and Nutrient Agar (DifcoTM).
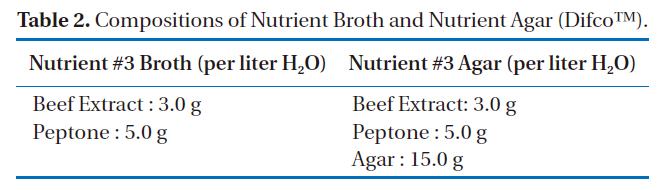
Compositions of Nutrient Broth and Nutrient Agar (DifcoTM).
[Table 3.] Composition of the liquid growth medium [12].
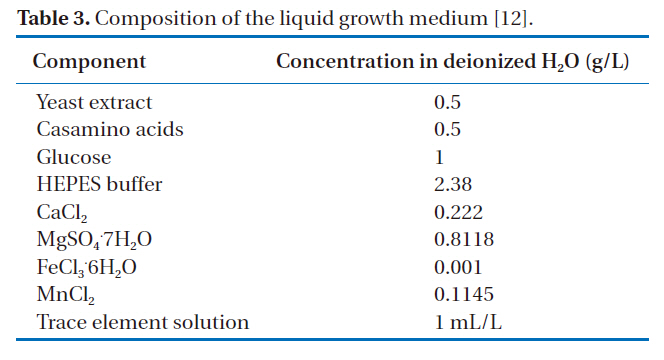
Composition of the liquid growth medium [12].
1240, Simadzu), was used as the indicator of the bacterial concentration [8, 13-14]. All chemicals in this study were purchased from Sigma-Aldrich Inc. and were of HPLC or analytical grade.
2.3. Batch experiments for the oxidation of Mn(II)
The oxidation of Mn(II) by
The Mn(II) concentration of the filtrate was analyzed according to Standard Methods [16], using a spectrophotometer (UVmini 1240, Simadzu), with the amount of Mn oxides in the liquid culture quantified by reaction with the reductive dye, Leucoberbelin blue I (LBB) [17]. Oxidized LBB is blue and the intensity of coloration is a function of the amount of Mn oxides having been reduced. The color intensity was measured for optical density at 618 nm using the spectrophotometer.
2.4. Characterization of the biogenic Mn oxides.
Mn oxides particles should be separated for examination of their properties by XRD and microscopy because organics and cell material accounted for more than 80% of the total resulting solid mass prior to the biological oxidation of Mn. The purification procedure aimed at ensuring the removal of organic materials (and absorbed anions and so on) that may interfere with the subsequent characterization. This involved a relatively harsh treatment to oxidize and dissolve the organic matter. The procedure reported by Mandernack et al. was adopted in this study, as described below [18].
1. Raw oxides in the suspension were collected in 40 mL vials and centrifuged for 20 min at 2000 rpm and room temperature. The supernatant and visible solid cell materials settled on the Mn oxides were discarded, with the solids washed by shaking with deionized (DI) water for 5 min. The above procedure was repeated 10 times.
2. Dissolution of the cell material using a 45 min sonication treatment with phenol, and subsequent 10 min sonication treatments with a phenol:chloroform (50:50, v/v) mixture, chloroform and a methanol:chloroform:water (12:5:3, v/v/v) mixture.
3. A 10-cycle DI water wash, as in step 1.
4. The suspension was acidified to pH 3, shaken for 30 min and centrifuged. The supernatant solution was discarded to eliminate any absorbed Mn (II).
5. A 10-cycle DI water wash, as in step 1.
6. Oxidation of the remaining solid organic material was acpositions complished by shaking for 4 hr in 0.17% NaClO solution.The high pH (>10) of this solution ensured the removal of absorbed anions.
7. A 10-cycle DI water wash, as in step 1.
For XRD analysis, the samples were oven-dried overnight at 60℃, homogenized with a mortar and pestle and subjected to an 18 kW X-Ray diffractometer (M18XHF-SRA, MAC Science). The samples were mounted on a holder and scanned over the range of 2θ = 5-90°. A field emission transmission electron microscope (FE-TEM, JEM-2100F, JEOL), operating at 200 kV, was used to acquire TEM images of the biogenic Mn oxides. For the TEM, the Mn oxides were dispersed in DI water, with a grain mount prepared by placing a drop of the suspension on a 3 mm Cu grid,with holey carbon film, and left to dry in the air before its introduction into the TEM. Scanning electron micrographs were obtained using a field emission scanning electron microscope(FE-SEM, LEO supRA55, Carl Zeiss) via energy dispersive X-ray spectroscopy (Genesis 2000, EDAX).
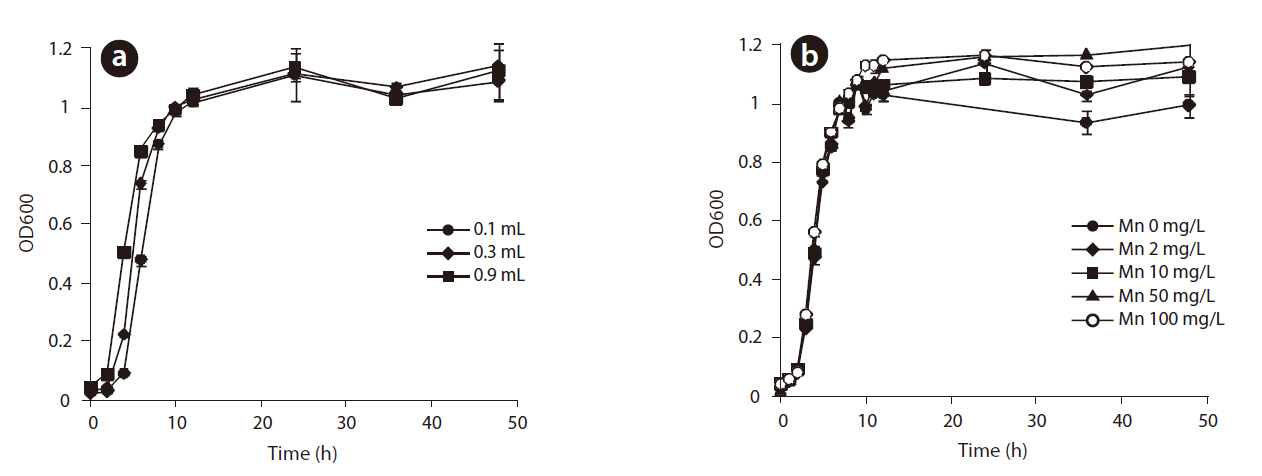
The growth of
to determine the experimental conditions for the oxidation of Mn(II) because the formation of Mn oxides occurs during different growth phases with different microbial strains. For example, the oxidation of Mn(II) takes place after the stationary phase with
Growth curves for
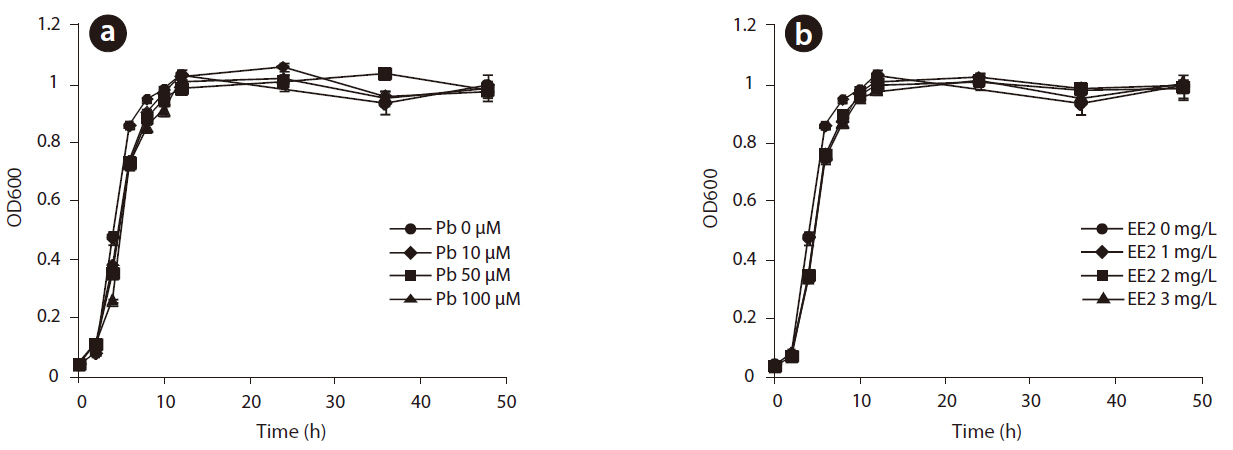
The growth of
3.2. The oxidation of Mn(II) by P. putida MnB1 and the effects of pH and temperature
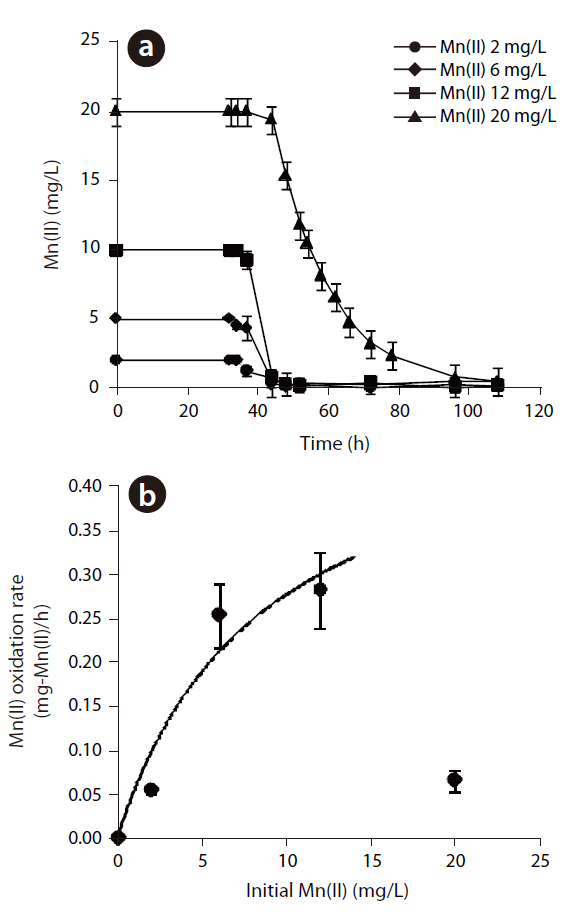
The Mn(II) concentrations during 108 hr of incubation with varying initial Mn(II) concentrations are shown in Fig 3. The oxidation of Mn(II) started after the stationary growth of
MnB1, as observed in the generation of Mn oxides by
In eq. (1), k is the maximum Mn(II) oxidation rate (mg-Mn/mg-cell?min)], KS the half velocity constant (mg-Mn/) and X the cell concentration (mg-cell/L), which was 391 mg/L, by measuring the suspended solids in the bacterial suspension. which were assumed to be constant during the oxidation of Mn(II). The k and Ks values were estimated to be 1.33x10-3 mg-Mn(II)/mg-cell?h and 8.81 mg-Mn/L, respectively. Based on these parameters,
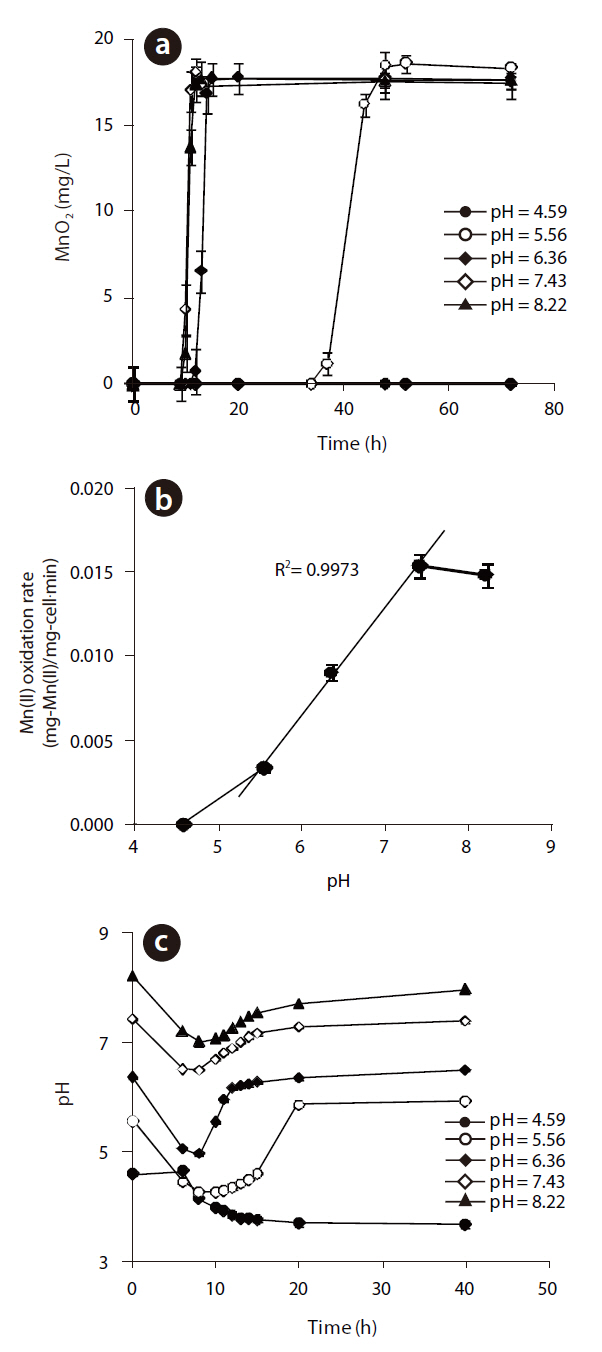
The MnO2concentrations with respect to reaction time at different initial pH are illustrated in Fig 4 (a). The maximum rate of MnO2 generation was observed at pH 7.43, but this was slightly lower at pH 8.22. However, when the initial pH was 5.56, the rate of biogenic Mn oxides generation was decreased significantly and lag phase increased dramatically. No Mn oxides were formed at an initial pH of 4.59. These results show that the Mn(II)-oxidizing enzyme was active at pH higher than 5.56. The rates of Mn(II) oxidation at various initial pH are shown in Fig 4(b). As the pH decreased from 8.22 to 5.56, the rate of Mn(II) oxidation for
to the original pH after the completion of Mn(II) oxidation. No pH recovery was observed at pH 4.59, when no Mn oxides were formed at all.
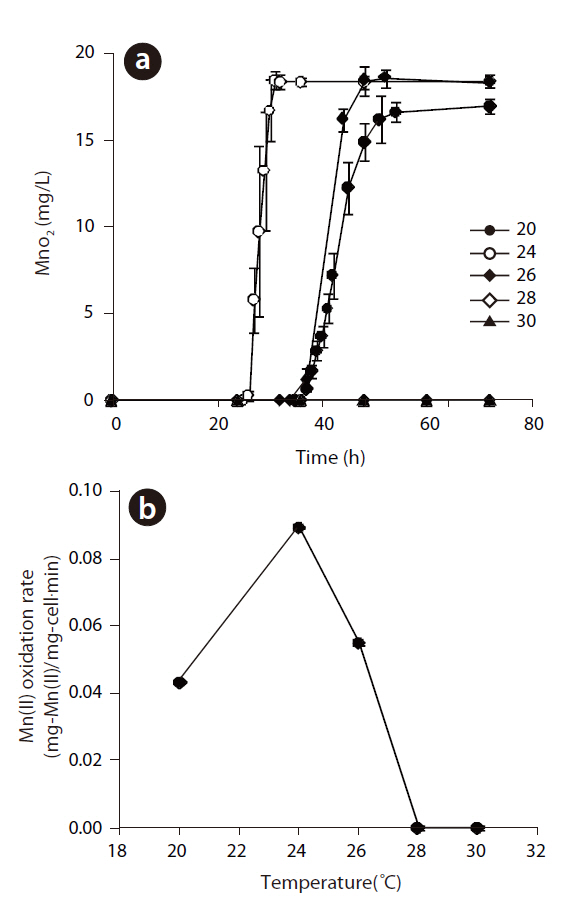
Manganese-oxidizing microorganisms are known to be both pH and temperature sensitive. The production of Mn(II) at different temperatures, ranging from 20 to 30℃, at pH 5.56 and an initial Mn(II) of 12 mg/L is shown in Fig 5 (a). The rate of Mn(II) oxidation as a function of the temperature is also shown in Fig 5(b). Mn(II) was not oxidized at 28 or 30℃, but Mn oxides formed under 26℃, with an optimum temperature of 24℃, with the shortest lag phase and highest rate of Mn(II) oxidation. In addition,The oxidation of Mn(II) by
3.3. Property of the biogenic Mn oxides
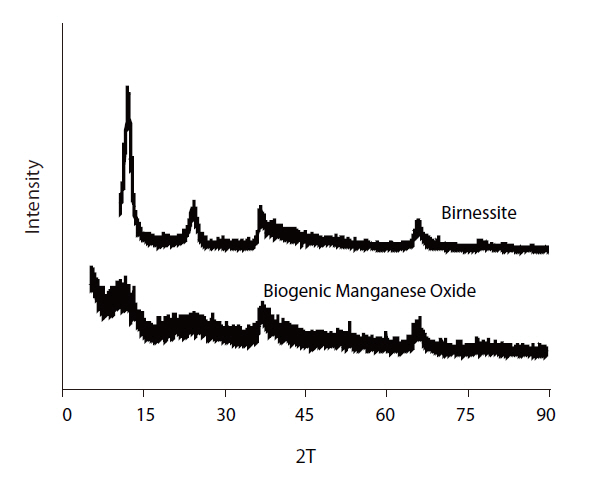
The XRD patterns of the biogenic Mn oxides formed by
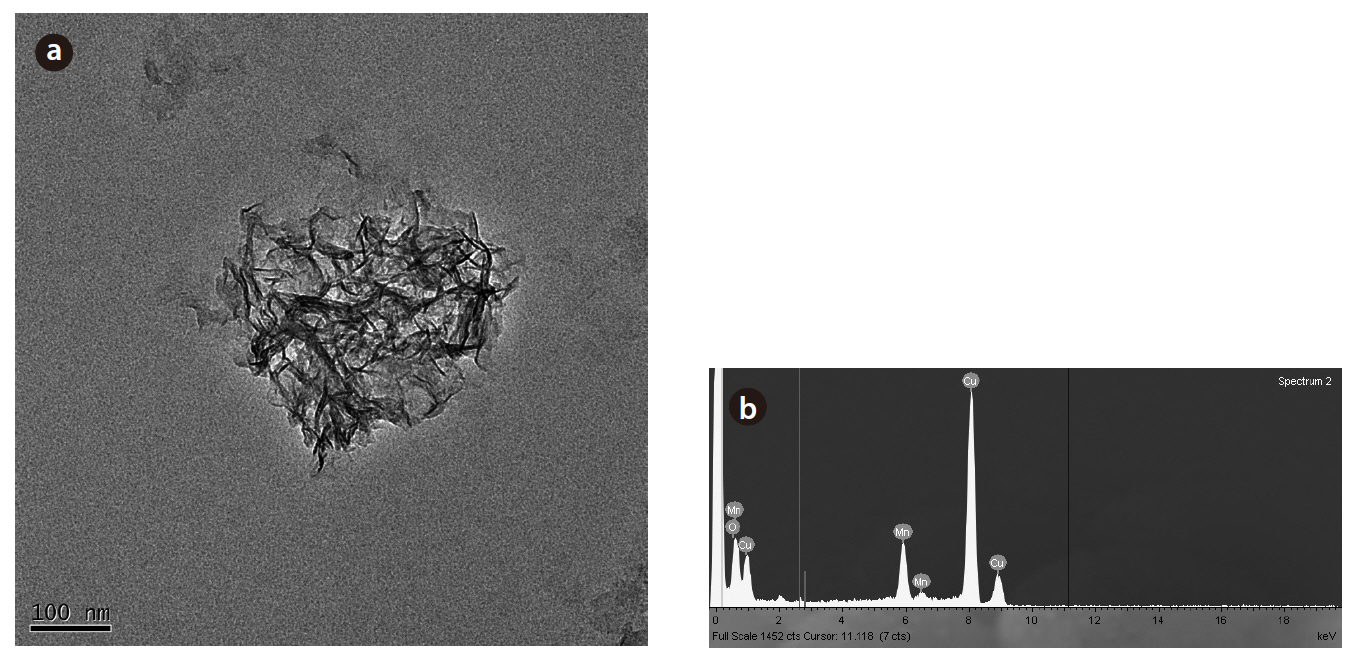
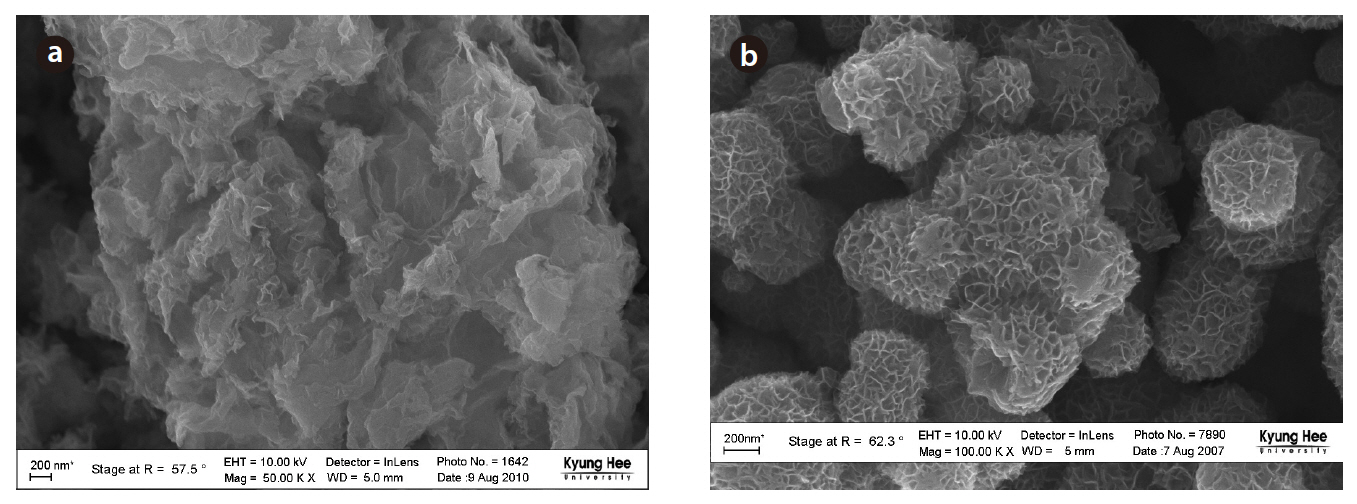
biogenic Mn oxides exhibited broader peaks of lower amplitude in this region. However, the XRD patterns of both the biogenic and abiotic Mn oxides showed small peaks at 2θ of 35 and 65°.These peaks were typical of varnadite, a fine-grained, poorly crystalline, natural Mn oxide phase found in the oxidized zone of Mn ore deposits, which is considered a major phase in ocean Mn nodules and other Mn oxide crusts and coatings [26]. The TEM image and EDS analysis of the biogenic Mn oxides showed the aggregation of small particles, with irregular shape, confirming the poor crystallinity (Fig 7). The SEM image also shows shape irregularity compared to birnessite (Fig 8).
Biogenic Mn oxides are generally known to be poorly crystalline compared to abiotic Mn oxides. Biogenic Mn oxides have intermediate crystallinity and reactivity, between those of synthetic phyllomanganates δ-MnO2 and acid birnessite, which are formed through the reduction of permanganate with MnCl2 or HCl, respectively [5, 12]. The X-ray Absorption Near Edge Structure (XANES), Extended X-ray Absorption Fine Structure (EXAFS) spectra and X-ray Diffraction (XRD) intensity data of the biogenic Mn oxides produced by the spores of marine
The growth of
ides were investigated to characterize the biogenic oxidation of Mn by
Poorly crystalline, nano-sized biogenic Mn oxides were formed in a system containing
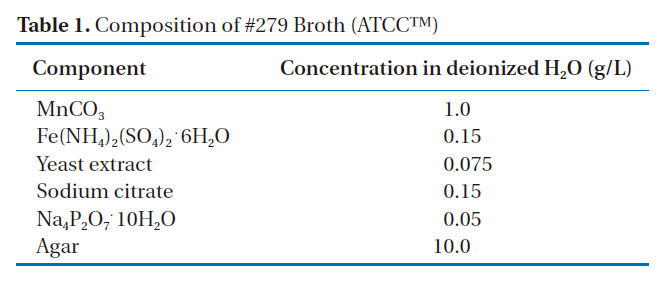
![Composition of the liquid growth medium [12].](http://oak.go.kr/repository/journal/10309/E1HGBK_2010_v15n4_183_t003.jpg)