Forest soils are often nitrogen-limited, and nitrogen input to forest soils can cause substantial changes in the structure and functions of a soil ecosystem. To determine the effects of nitrogen input on methane oxidation and the microbial enzyme activities, manipulation experiments were conducted using nitrogen addition to soil samples from Mt. Jumbong. Our findings suggested that the addition of nitrogen to the soil system of Mt. Jumbong did not affect the microbial enzyme activities. Conversely, the addition of nitrogen affected the rate of methane oxidation. Inorganic nitrogen in soils can inhibit methane oxidation via several mechanisms, such as substrate competition, toxic effects, and competition with other microbes, but the inhibitory effects are not always the same. In this research, seasonal changes were found to produce different inhibitory factors, and these different responses may be caused from differences in the methantrophic bacteria community structure.
Nitrogen is one of the limiting factors for many of the reactions within terrestrial ecosystems. Especially, forest soils are well known to be nitrogen deficient ecosystems. Therefore, nitrogen input into forest soils can cause many reaction changes. The input of nitrogen from atmospheric to terrestrial ecosystems has continuously increased over the past 300 years due to the increased use of fertilizers and fossil fuels. Nitrogen input to forest soils makes the availability of nitrogen much higher than in soils with no nitrogen addition; therefore, the primary production of plant is also stimulated, which alters the nitrogen cycle. In addition, extra nitrogen changes the C/N/P ratio in soils,which then causes changes in other nutrient cycles[1-3]. Other studies on the effects of nitrogen input on the microbial activities and community have been performed [4-7].
Nitrogen input to forest soils can also have an inhibitory effect on methane oxidation, which is an important function of forest soils. Methane is one of the most potent greenhouse gases,accounting for approximately 15% of the current greenhouse effects. One of the largest sinks for methane is soils, where methane can be oxidized by methanotrophic bacteria. The amount of methane oxidized by soil methanotrophic bacteria is estimated to be between 10 and 30 Tg yr-1[8, 9]. Forest soils are well known to have a high ability for methane oxidation. Previous studies on methane oxidation by forest soils have shown the process is mainly controlled by the soil temperature [10-12] and moisture[11, 13-15], and by human activities, such as land use changes[16] and nitrogen input [17]. Nitrogen, especially in the form of ammonium ions, has drawn much attention due to its inhibitory effect on methane oxidation [18-20]. Recent studies have suggested that not only ammonium ions, but nitrate ions have inhibitory effects on methane oxidation. For example, Xu and Inubush [21] and Reay and Nedwell [22] showed a negative correlation between nitrate concentrations and the rates of methane oxidation in temperate forest soils.
In this research, a manipulation experiment was conducted with forest soils to determine the effects of nitrogen input on methane oxidation and the microbial enzyme activities.
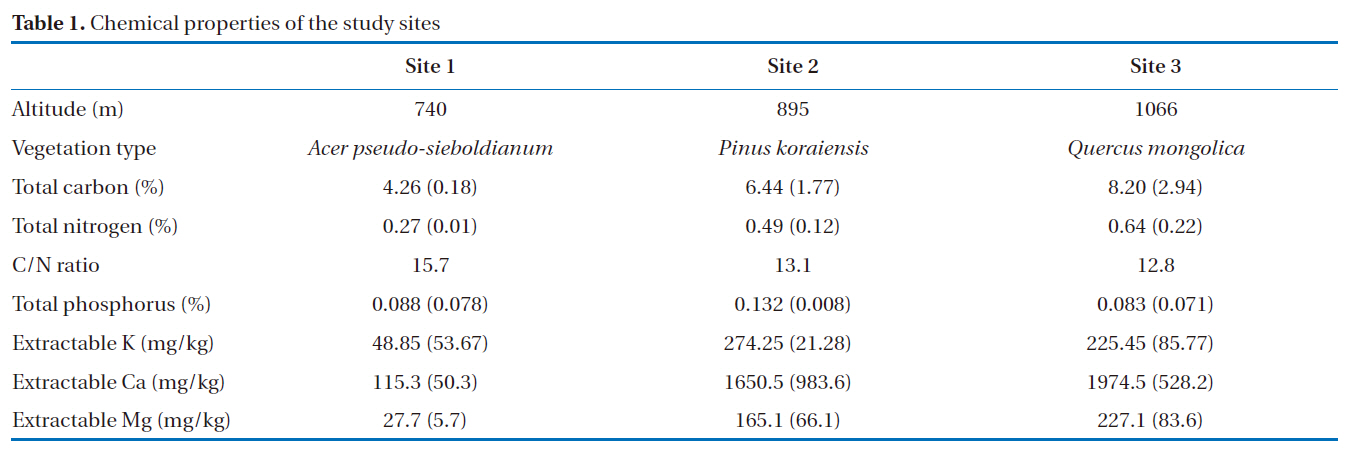
These experiments were conducted in December 2007 and July 2008. Soil samples were collected from Mt. Jumbong(38º02´N, 128º26´E), South Korea. Mt. Jumbong is a hard-wood forest located at the southern-most side of the Mt. Seorak national park, Kangwon Province. The site is included in a UNESCO Biosphere Reserve and designated as a Natural Forest Reserve by the Korea Forest Service. The soil is classified as sandy clay loam (sand: 49%, silt: 24%, clay: 27%). Soil samples were collected at 3 different locations with different elevations (Table 1), with 2 replicates collected at each site to a depth of 5 cm from the soil surface, and the samples were sieved (2 mm) in the laboratory and then combined in equal amounts.
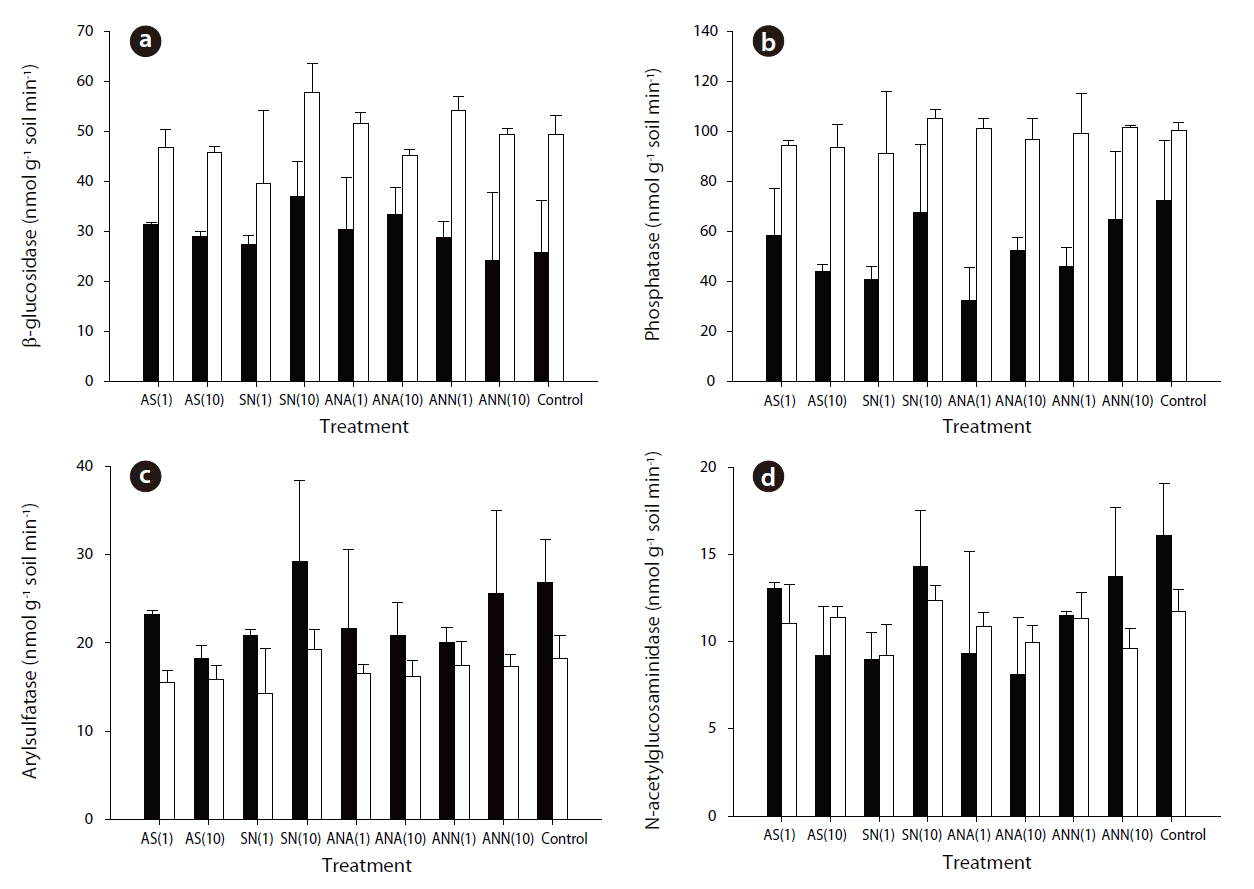
Thirty grams of the composite soil samples were placed into a 120 mL serum vial, with 12~150 μg of N applied to the soil samples. The amount of N added was based on the estimation of N deposition reported by Park et al. [23] and Park and Lee [24]. Park et al. [23] estimated the rate of dry deposition using a simplified chemical model; whereas, Park and Lee [24] monitored the amounts of wet nitrogen deposition from 10 sites over South Korea. To determinate the response of methane oxidation and enzyme activities to nitrogen addition to the soil samples, 4 different concentrations of NH4NO3 were added to the soil samples (2.40, 4.74, 24.0 and 47.4 ng g-1 soil: ANN(1), ANA(1), ANN(10), ANA(10)). To remove the effects of other salts (i.e., Na+, SO4 2-) on methane oxidation and enzyme activities, separate treatments of NaNO3 were prepared (1.20 and 11.99 ng g-1 soil: SN(1) and SN(10)) and (NH4)2SO4 (2.37 and 23.69 ng g-1 soil: AS(1) and AS(10)). These soils were incubated for two weeks.
After incubation for one day to allow for stabilization, the vials were sealed with a septum and injected with a high concentration of methane (300 ppmv). Gas sampling was conducted every day for two weeks using a gas tight syringe. The gas samples were analyzed in the laboratory using GC-FID (PORAPAK Q 80/100 column, the detector operated at 150℃, carrier gas: N2). The methane oxidation rates were calculated using the following equation [25]:
where FM is the flux of methane gas (μg CH4 g soil-1 min-1);
the rate of change in the methane concentration (㎛ol mol-1 min-1) over time; V the chamber headspace volume (m3); MCH4 the molecular weight of methane (16 g mol-1) and Vmol the volume of a mole of gas at a certain temperature (m3 mol-1).
2.4. Enzyme Activities in Soils
The enzyme activities were measured at the end of the incubation period. Methylumbelliferyl (MUF)-substrates were used as model substrates for the soil enzymes. MUF-β-D-glucoside(MUF-G), MUF-N-acetylglucosaminide (MUF-N), MUF-phosphate (MUF-P) and MUF-sulfate (MUF-S) were used for β-glucosidase, N-acetylglucosaminidase, phosphatase and arylsulfatase, respectively. Soil samples of 1.5 g were amended with 5 ml of the substrate (400 ㎛ol). After 60-min incubation at 20℃, the fluorescence of the supernatant was determined using a TD-700 fluorometer at emission and excitation wavelengths of 450 and 330 nm, respectively. For each sample, a calibration curve was prepared using 0~200 ㎛ol of the MUF-free acid to account for the quenching effect and soil absorption [26].
To determine the effects of the addition of nitrogen and seasonal variations, the methane oxidation rates and activities were analyzed using a two-way ANOVA. Non-parametric correlation was used to determine the relationships between environmental factors and the methane oxidation rates.
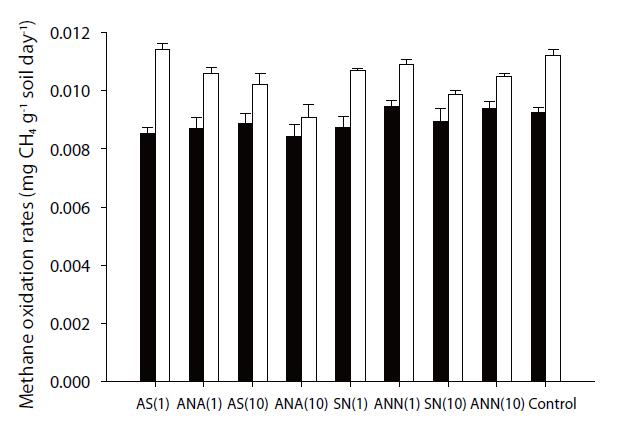
The methane oxidation rates varied from 11.29 to 16.23 ng g-1 soil day-1 (Fig. 1), and those during winter were significantly higher than those during summer (
[Table 1.] Chemical properties of the study sites
Chemical properties of the study sites
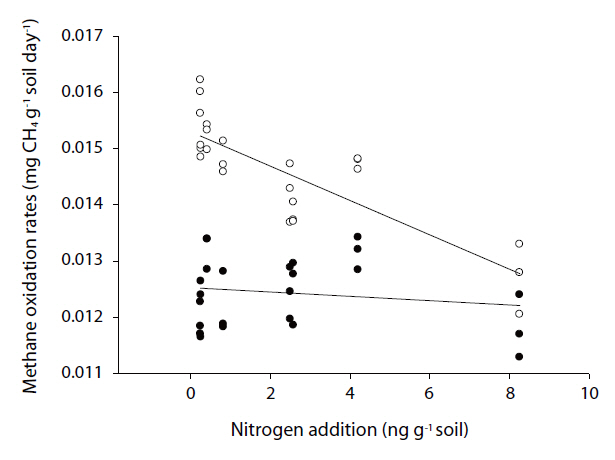
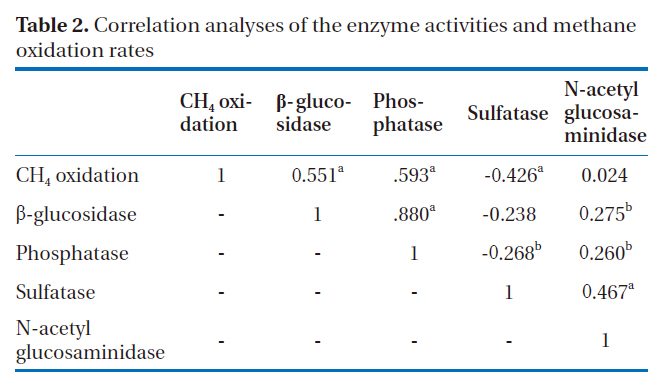
relation with the amount of nitrogen added (r=0.838, p=0.000,n=30). However, there was no significant relationship with the oxidation rates during summer (Fig. 3).
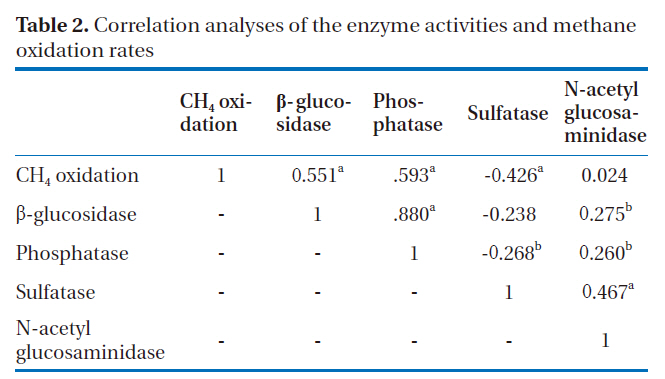
The phosphatase and N-acetylglucosaminidase activities exhibited the highest and lowest enzyme activities, respectively.The average values of β-glucosidase, phosphatase, arylsulfatase and N-acetylglucosaminisase during summer were 29.58, 53.12, 22.93 and 11.53 nmol g-1 soil min-1, respectively. In winter, the average values were 48.88, 98.26, 16.74 and 10.83 nmol g-1 soil min-1, respectively. The β-glucosidase, phosphatase and N-acetylglucosaminidase activities during winter were significantly higher than those during summer (
[Table 2.] Correlation analyses of the enzyme activities and methane oxidation rates
Correlation analyses of the enzyme activities and methane oxidation rates
The effects of the addition of nitrogen on the microbial activities and methane oxidation rates in forest soils were investigated. Due to nitrogen being a limiting factor, there has been extensive research on the topic of its impacts on forest ecosystems, including nitrogen addition and nutrient cycling, primary production and the microbial response [7, 10, 12]. However, no distinct differences have been observed in the enzyme activities between samples on the addition of nitrogen and the controls. The nitrogen cycle in forest soils is closely connected with other components, such as carbon and phosphorus. Especially, the C/N ratio in forests soils is important in explaining the carbon and nitrogen cycles. According to Michel and Matzner [27], different responses were found on the addition of nitrogen in soils with various C/N ratios. Forest soils with a low C/N ratio showed no response on the addition of nitrogen, but higher C/N ratios stimulated enzyme activities. From the experimental results of Michel and Matzner [27], the lower C/N ratio was 17.9, but the C/N ratios at our sites varied from 12.8 to 15.7, i.e. much lower.According to this comparison, it would appear that our forest
ecosystem might be controlled by the availability of carbon.
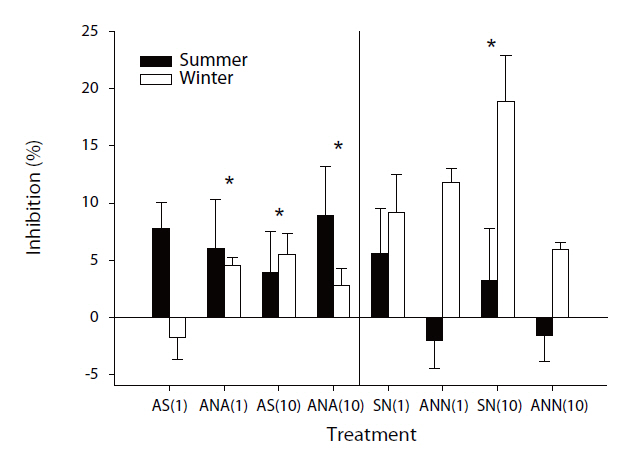
However, inhibitory effects were observed on the methane oxidation rates on the addition of nitrogen, which varied from -2.05 to 18.89%. Some samples exhibited negative values due to stimulation from the addition of nitrogen, but most samples showed the inhibitory effects. The effects of inorganic nitrogen on methane oxidation in forest soils have previously been investigated[17, 18, 20-22]. Methanotrophic bacteria, which can oxidize methane to methanol, have also shown the ability to oxidize ammonium ions [19]. Therefore, the existence of ammonium ions can inhibit methane oxidation, which has been reported in many studies [17, 21, 28]. Recently, there have been investigations on the inhibition of nitrate on methanotrophs, but the inhibitory mechanisms are yet to be revealed. There are some hypotheses aimed at better understanding this occurrence, with one being the possible toxicity of NO2- towards methanotrophic bacteria. According to Dunfield and Knowles [29], nitrite acted as a toxicant to methanotrophic bacteria. Nitrate can be reduced to nitrite due to anaerobic micro-sites in otherwise aerobic soil. In these spots, nitrate can be reduced to nitrite, which will affect methane oxidizers; another possibility is competition with other microbes. Since methanotrophic bacteria are heterotrophic, they are not subject to environmental factors [19], but other microbes are easily are affected by environmental factors, such as temperature, pH and nutrient availability. Therefore, the addition of nitrogen might stimulate other microbes, which can out-compete the methanotrophic bacteria and; thereby, inorganic nitrogen can inhibit methane oxidation.
The effects of nitrogen on methane oxidation were found to be seasonally different, with those during winter inhibited by the addition of nitrogen, but this effect was not shown during summer. These differences might come from the different community structures of methanotrophic bacteria. Therefore, the community structure of methanotrophic bacteria was analyzed using a DNA-based experiment. The results showed the community structures distinctly differed with season. Especially, the community structure during winter was significantly different than that during summer (Unpublished data). Therefore, the response to the addition of nitrogen can differ.
The addition of nitrogen at Mt. Jumbong did not affect the microbial enzyme activities. Since, the C/N ratio in this area was found to be low, the addition of nitrogen exhibited little effect.Conversely, the addition of nitrogen affected the methane oxidation rates. Inorganic nitrogen in soils could inhibit the methane oxidation rate via several mechanisms, such as substrate competition, toxic effects and competition with other microbes. However, the inhibitory effects are not always the same. In our research, they differed between seasons. These different responses might come from the differences in the methantrophic bacteria community structure.