The objective of this study was to investigate microbial removal using layered double hydroxides (LDHs) and iron (hydr)oxides (IHs) immobilized onto granular media. Column experiments were performed using calcium alginate beads (CA beads), LDHs entrapped in CA beads (LDH beads), quartz sand (QS), iron hydroxide-coated sand (IHCS) and hematite-coated sand (HCS). Microbial breakthrough curves were obtained by monitoring the effluent, with the percentage of microbial removal and collector efficiency then quantified from these curves. The results showed that the LDH beads were ineffective for the removal of the negatively-charged microbes (27.7%at 1 mM solution), even though the positively-charged LDHs were contained on the beads. The above could be related to the immobilization method, where LDH powders were immobilized inside CA beads with nano-sized pores (about 10 nm); therefore, micro-sized microbes (E. coli = 1.21 ㎛) could not diffuse through the pores to come into contact with the LDHs in the beads, but adhere only to the exterior surface of the beads via polymeric interaction. IHCS was the most effective in the microbial removal (86.0% at 1 mM solution), which could be attributed to the iron hydroxide coated onto the exterior surface of QS had a positive surface charge and, therefore, effectively attracted the negatively-charged microbes via electrostatic interactions. Meanwhile, HCS was far less effective (35.6% at 1 mM solution) than IHCS because the hematite coated onto the external surface of QS is a crystallized iron oxide with a negative surface charge. This study has helped to improve our knowledge on the potential application of functional granular media for microbial removal.
Contamination of surface water and groundwater by microbial pathogens is a serious environmental problem, causing deterioration of the quality of drinking water resources and posing a great threat to human health. Inadequate sanitation causes waterborne diseases, leading to a great number of deaths around the world [1]. Understanding of microbial interactions with granular media is important with respect to the removal of microorganisms in water filtration systems. In granular media,the movement of microorganisms is mainly controlled by advective-dispersive transport and attachment to solid matrices [2]. The deposition of microorganisms on granular media is affected by the properties of the granular media (e.g., surface charge and grain size), solution chemistry (e.g., ionic strength, pH and ionic composition) and characteristics of the microorganisms (e.g., cell surface charge and hydrophobicity) [3, 4].
Point-of-use (POU) water treatment alternatives are of considerable interest as they provide safe drinking water and subsequently prevent water-related diseases. POU treatment could be directly applied to treat the water used at a single tap within a home [5-9]. Layered double hydroxides (LDHs) could be used in POU treatment systems to remove microbial pathogens. LDHs are a class of nanostructured anionic clays, consisting of positively charged brucite-like sheets, which are balanced by the intercalation of anions in the hydrated interlayer regions [10]. Because of their high surface area, large anion exchange capacity,and good thermal stability [11-13], LDHs have been studied for their potential application to microbial removal [14, 15]. Iron (hydr)oxides (IHs) could also be applied to remove microorganisms. Through the modification of the surface charges on granular media with IHs, the microbial removal in water filtration systems could be enhanced [16-18]. Further experiments and quantitative analyses are required to improve our knowledge regarding the microbial removal using these hydroxides.
The objective of this study was to investigate the microbial removal using LDHs and IHs immobilized onto granular media with three sets of column experiments. The first experiments were performed on calcium alginate beads (CA beads) and LDHs entrapped in CA beads (LDH beads), while the second and third experiments were carried out on iron hydroxide-coated sand (IHCS) and hematite-coated sand (HCS). Microbial break-through curves (BTCs) were obtained by monitoring the effluent, with the percentage of microbial removal and the collector efficiency quantified using the curves.
Transmission electron microscopy (JEM 1010; JEOL, Tokyo, Japan) was used to obtain images of the
All chemicals used for the experiments were purchased from Sigma Aldrich. LDH powders were prepared by co-precipitating mixtures of magnesium nitrate [Mg(NO3)2·6H2O] and aluminum nitrate [Al(NO3)3·9H2O]. A 700 mL solution (Mg/Al molar ratio = 2) of Mg(NO3)2·6H2O (1 mol) and Al(NO3)3·9H2O (0.5 mol) was added drop wise, using a peristaltic pump (QG400; Fasco, Springfield, MO, USA) at a rate of 3 mL/min, into 1,000 mL of an alkali solution (pH = 13) of sodium hydroxide (NaOH) and sodium carbonate (Na2CO3), with intensive stirring, at room temperature. The resulting precipitates were oven-dried at 65℃ for 18 hours, and then washed thoroughly with deionized water to remove excess sodium, with the final suspensions centrifuged at 8,500 rpm for 20 minutes. The washed precipitates were ovendried again at 65℃ for 24 hours and then pulverized in a ball mill. The Mg-Al LDHs used for the preparation of the LDH beads were finally obtained via calcination at 573 K for 24 hours in an electric muffle furnace (C-FMA; Vision Lab, Seoul, Korea).
The LDH beads were prepared by entrapping a powdered form of Mg-Al LDHs in a calcium alginate gel. Two grams of sodium alginate powder were added to 200 mL of deionized water at room temperature to prepare a sodium alginate solution. The desired amount of LDHs (8% w/v) was then added to 200 mL alginate solution, with intensive stirring, to obtain a homogeneous suspension. The suspension, contained in a plastic syringe (outlet diameter = 2 mm), was allowed to drop into a stirred reservoir containing 500 mL of 0.3M calcium chloride (CaCl2) solution in order to form 4.15 ± 0.23 mm (n = 90) spherical LDH beads. The beads were allowed to cure in the same CaCl2 solution for 24 hours, with stirring, and then rinsed with deionized water to remove any excess Ca2+.
Quartz sand (QS) was also used in the experiments (Jumunjin Silica, Kangreung, Korea). Mechanical sieving was conducted with US Standard Sieves Nos. 35 and 10. Sand fractions, with a grain size of 0.5-2.0 mm and a mean diameter of 1.0 mm, were used in the experiments. Before use, the sand was washed twice using deionized water to remove impurities from the surface,with the wet sand autoclaved for 20 minutes at 17.6 psi, cooled to room temperature, and then oven-dried at 105℃ for 1-2 days. For the preparation of the IHCS, FeCl3?6H2O (5.5 g) was dissolved in deionized water (100 mL), with the solution pH adjusted using 6 M NaOH. The QS (200 g) was added to FeCl3?6H2O solution and then mixed in a rotary evaporator (90℃, 80 rpm, 20 minutes) to remove the water in the suspension by heating (Hahnvapor; Hahnshin Scientific Co., Seoul, Korea). The coated sand was dried at 150℃ for 6 hours, washed with deionized water and then dried again under the same conditions. HCS was prepared following the same procedures used for the IHCS. The coated sand was thermally treated at 700℃ for 24 hours, in an electric muffle furnace (C-FMA; Vision Lab), to obtain HCS.
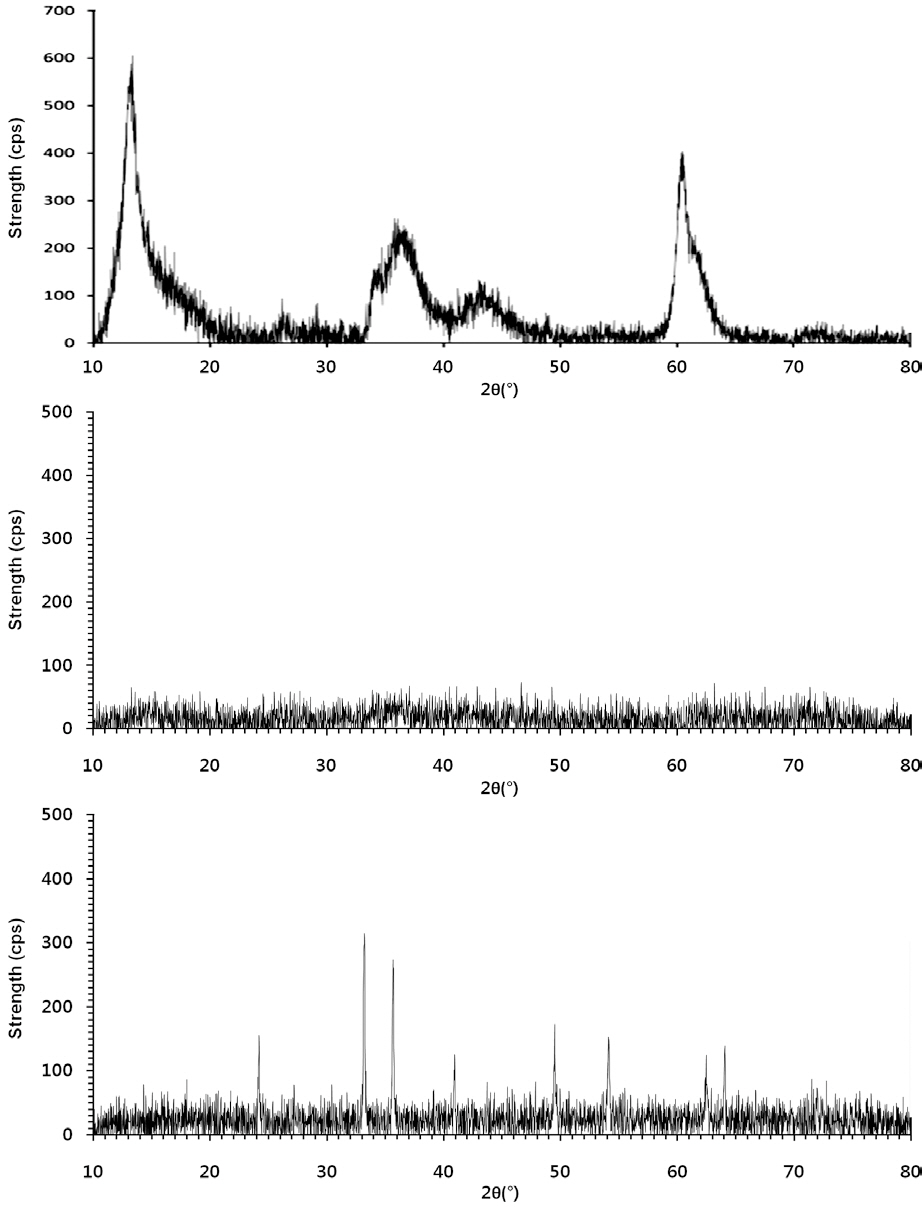
The powdered forms of the LDHs and IHs (Fig. 1) were characterized using powder X-ray diffractometry (XRD, D5005; Bruker AXS, Berlin, Germany), using Cu Kα radiation. The granular
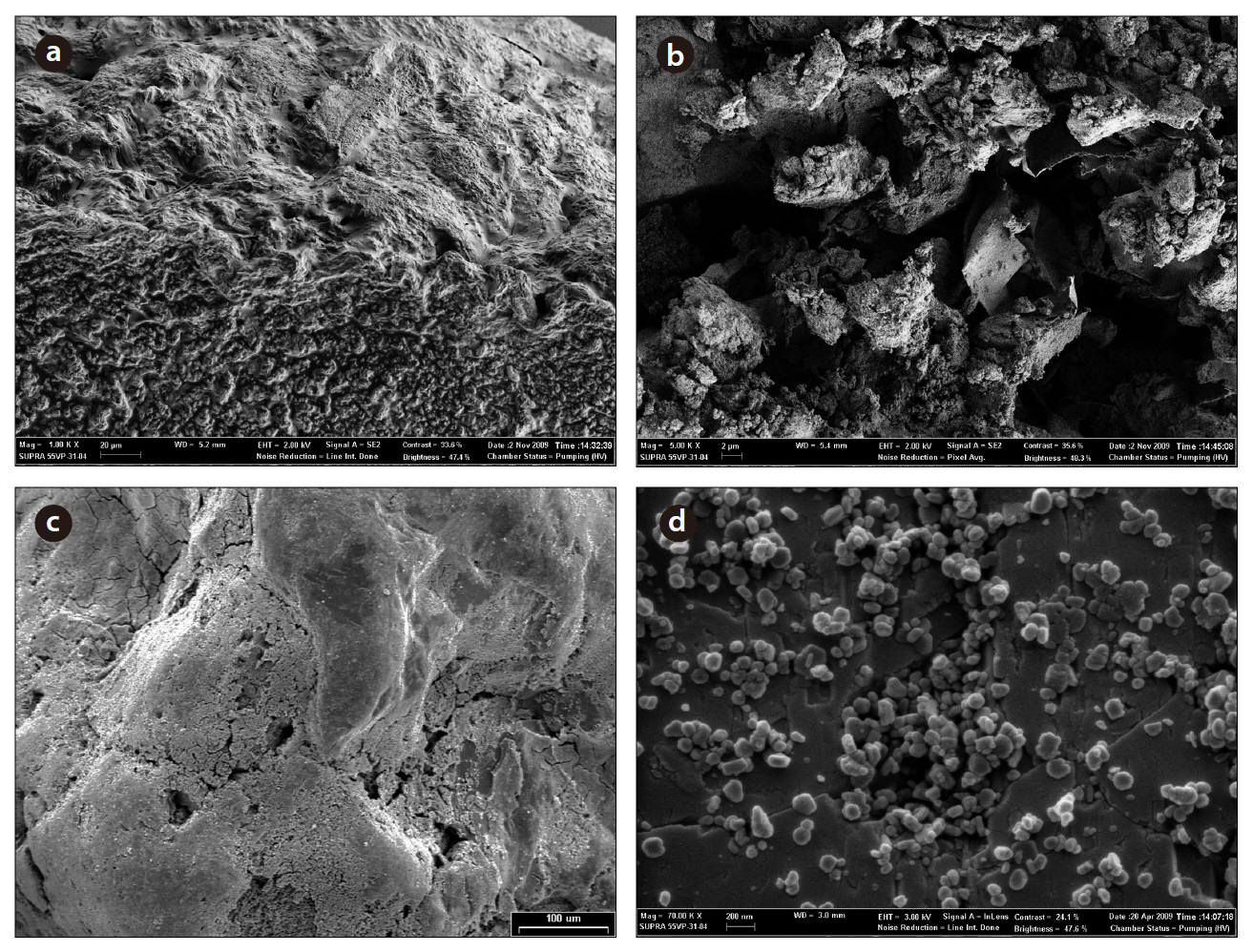
media were observed and examined using X-ray microanalysis (Fig. 2). The granular media were mounted on metal stubs using carbon tape and sputter-coated with platinum (approximately 20 nm in thickness). Specimens were examined with a field emission scanning electron microscope (FESEM, Supra 55VP; Carl Zeiss, Oberkochen, Germany), at an accelerating voltage of 3 kV.
Column experiments were conducted using Plexiglas columns, with an inner diameter of 2.5 cm and a height of 10 cm, packed with the granular media. All the experiments were performed in duplicate (Table 1). The column was connected to a HPLC pump (Series II; Scientific Systems, Inc., Woburn, MA,USA), operating at a rate of 0.3 mL/min. Before the microbial injection, the packed column was flushed upward with 15-20 pore volumes of NaCl solution (1, 10 and 100 mM) to achieve steady state flow conditions.
Assuming negligible microbial growth and decay, the onedimensional microbial transport can be described as:
where
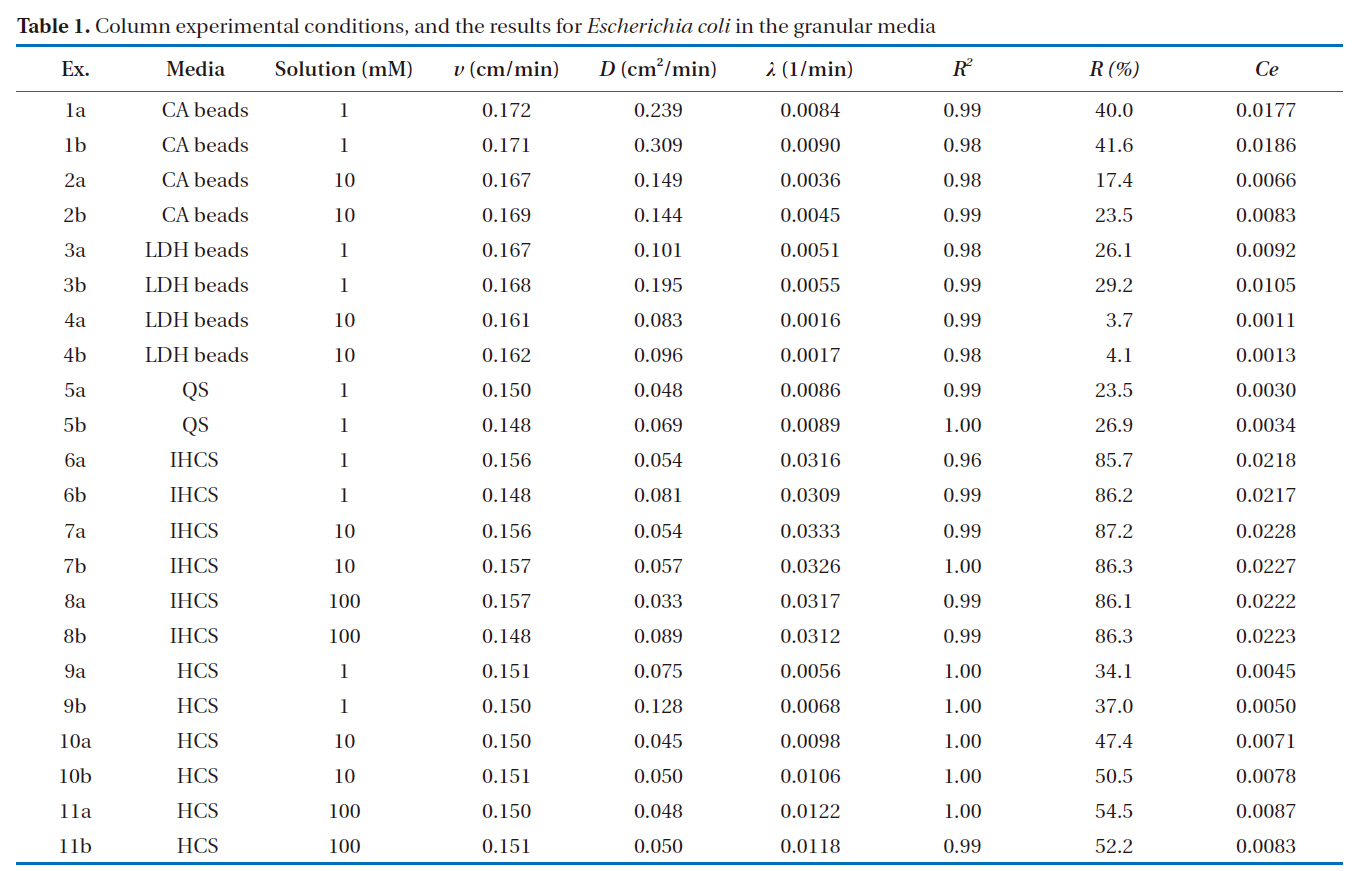
[Table 1.] Column experimental conditions and the results for Escherichia coli in the granular media
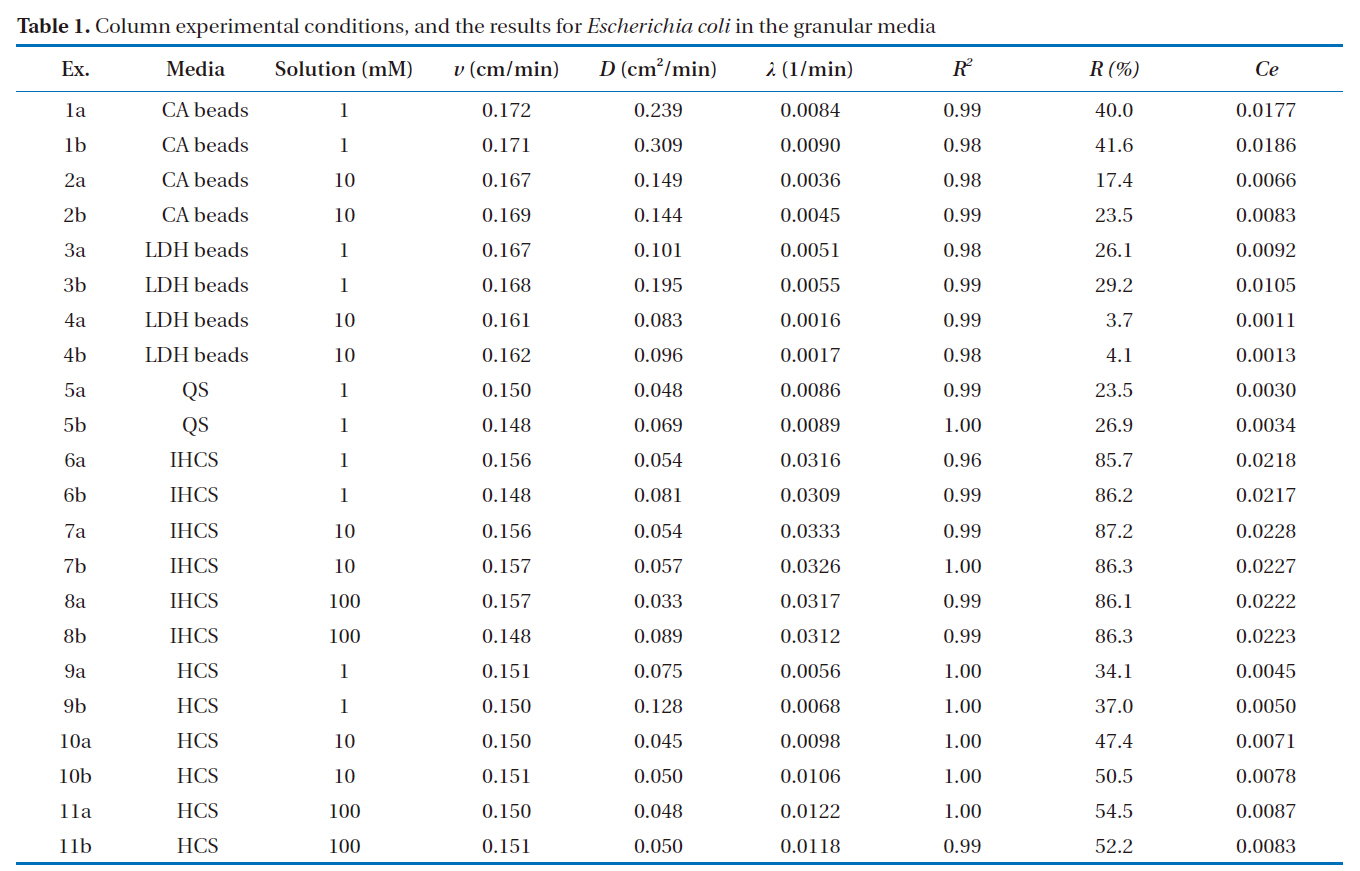
Column experimental conditions and the results for Escherichia coli in the granular media
tained by fitting the CXTFIT code [19] to the breakthrough data. The percentage of microbial removal (R) in the effluent was quantified by the following equation:
where
From colloid filtration theory, the removal of biocolloids/colloids from suspension by attachment to a collector can be described by the collector efficiency (
where η is the collision efficiency, which is the probability that a colloidal particle approaching a collector collides with the collector, and α the sticking efficiency, which is the probability that the particle colliding with the collector sticks to that collector. The collision efficiency is calculated using the following Eq. [21]:
where
where
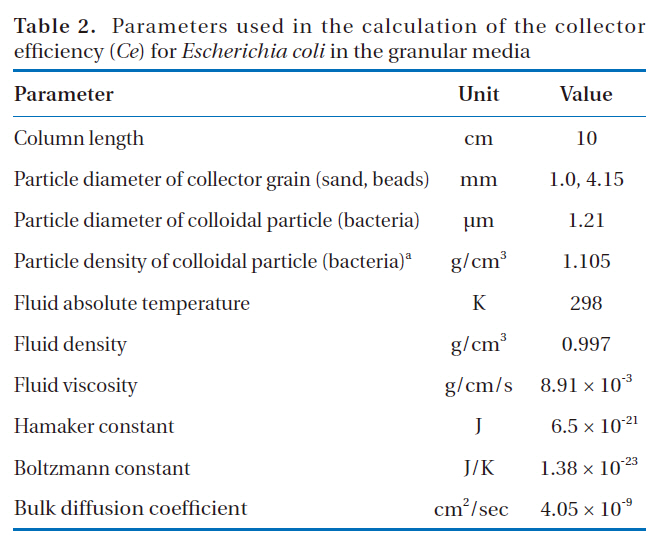
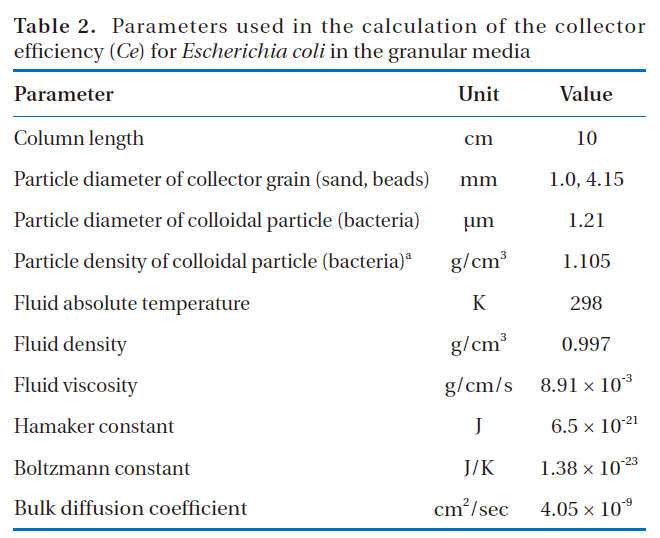
Parameters used in the calculation of the collector efficiency (Ce) for Escherichia coli in the granular media
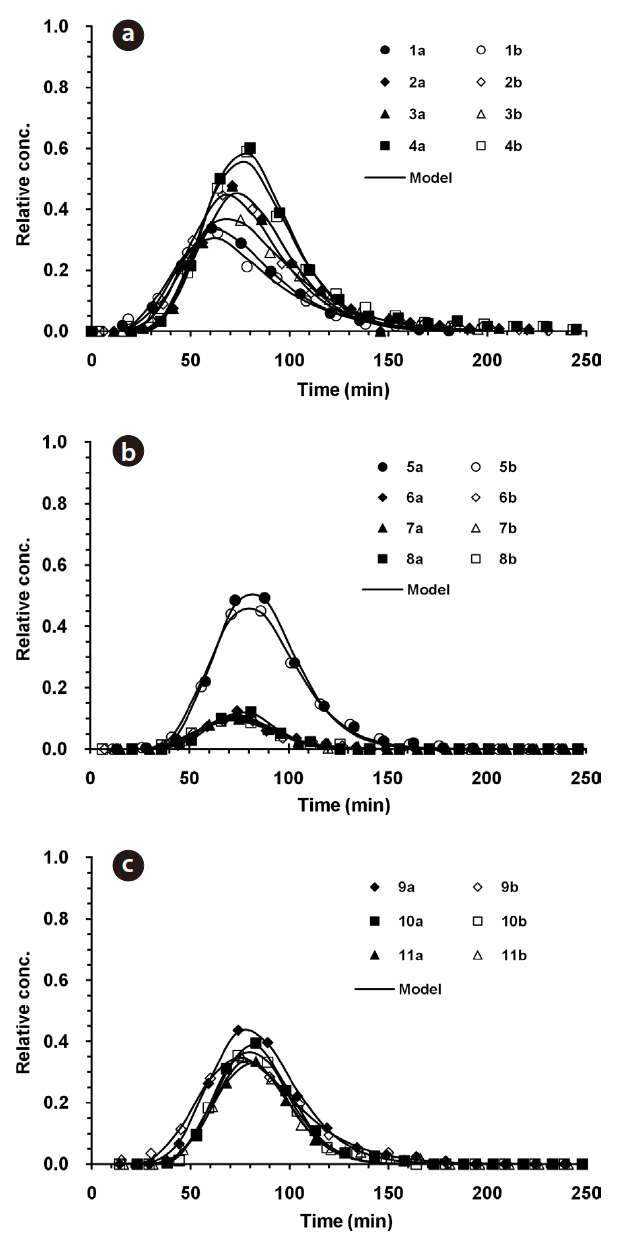
The microbial BTCs obtained from the column experiments using the granular media are presented in Fig. 3. With the CA beads and LDH beads (Fig. 3a), the BTCs had well defined single peaks, with different relative peak concentrations depending on the granular medium and ionic strength. The relative peak concentrations ranged from 0.323 to 0.601, with the lowest obtained for 1 mM solution and alginate beads (Ex. 1), and the highest with 10 mM solution and LDH beads (Ex. 4). The transport pa-
rameters (
The microbial BTCs from the experiments with QS and IHCS are given in Fig. 3b. The BTCs also had well-defined single peaks, with the relative peak concentrations ranging from 0.092 to 0.493, with the highest obtained for 1 mM quartz sand (Ex. 5). The values of
3.2. Microbial Removal Percent and Collector Efficiency
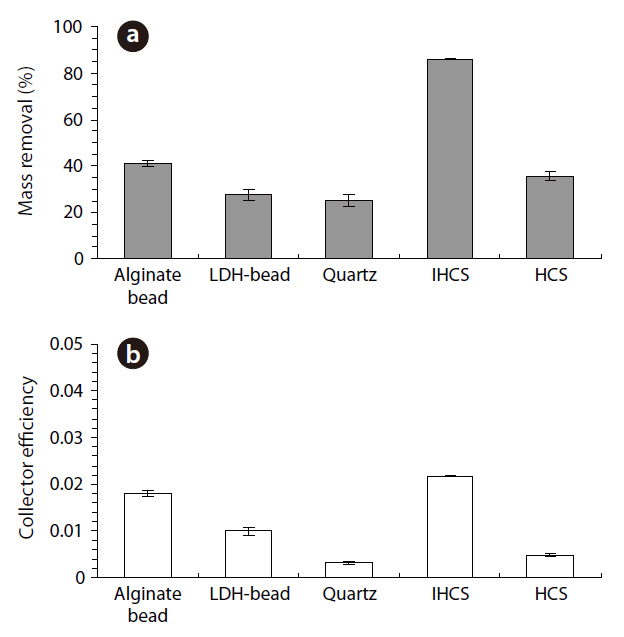
The percentage microbial removal and collector efficiency with 1 mM solution for the granular media are presented in Fig. 4. As shown in Fig. 4a, the average percentage removal (R) was highest for IHCS and lowest for QS. With the CA beads, the average percentage removal was 40.8% (1a = 40.0, 1b = 41.6), but 27.7% with the LDH beads (3a = 26.1, 3b = 29.2). The average percentage removal for QS was 25.2% (5a = 23.5, 5b = 26.9), which was lower than those for CA beads and LDH beads. With the IHCS, the average percentage removal was 86.0% (6a = 85.7, 6b = 86.2), which was about 3.4 times higher than that for QS. The average percentage removal with HCS was 35.6% (9a = 34.1, 9b = 37.0).
As presented in Fig. 4b, the average collector efficiency (Ce) was lowest with QS and highest with IHCS. With the CA beads, the average collector efficiency was 0.0181 (1a = 0.0177, 1b =0.0186), which was about two times higher than that (= 0.0099) of the LDH beads (3a = 0.0092, 3b = 0.0105). The average collector efficiency for QS was 0.0032 (5a = 0.0030, 5b = 0.0034).With the IHCS, the average collector efficiency was 0.0218 (6a =0.0218, 6b = 0.0217), which was one order of magnitude higher than that of the QS. The average collector efficiency with HCS was 0.0048 (9a = 0.0045, 9b = 0.0050).
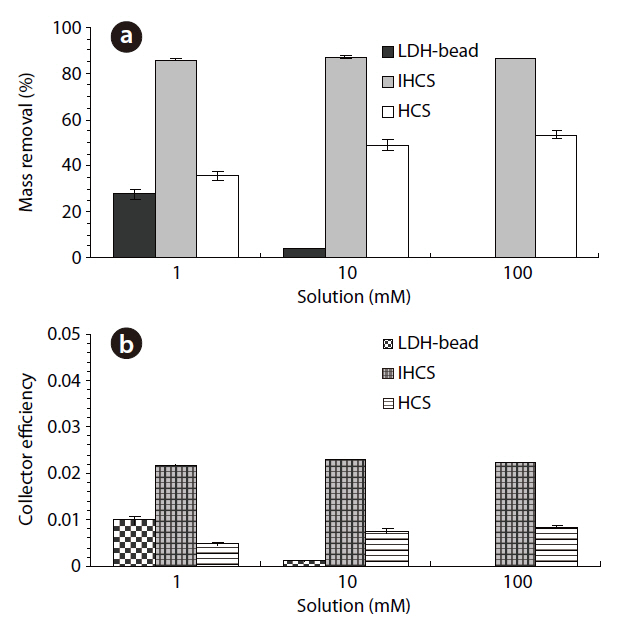
The percentage microbial removal and collector efficiency with the various solutions (1, 10 and 100 mM) are presented in Fig. 5. As shown in Fig. 5a, the average percentage removal with the LDH beads decreased from 27.7 to 3.9% (4a = 3.7, 4b= 4.1) on increasing the ionic strength from 1 to 10 mM. Note:no column result was obtained for LDH beads in 100 mM solution due to unstable behavior of the LDH beads. When NaCl was added to adjust the ionic strength, the Na+ ions partially displace Ca2+ ions from the alginate hydrogel beads [23]. With the IHCS, the average percentage removal remained constant at around 86.0%, even though the ionic strength was increased from 1 to 100 mM. Meanwhile, the average percentage removal with the HCS gradually increased from 35.6 to 53.4% (11a = 54.5, 11b =52.2) with increasing ionic strength from 1 to 100 mM (Table 1).
As shown in Fig. 5b, the average collector efficiency (
3.3. Microbial Removal in Granular Media
Sodium alginate, a water-soluble salt of alginic acid, is an anionic linear polysaccharide composed of repeated (1-4)-linked D-mannuronic and L-glucuronic acid units. Calcium ions can chelate carboxylate groups, make crosslinks between chains, and form an insoluble hydrogel [24]. CA beads are an anionic biopolymer, with a point of zero charge (pHpzc) of 6.5[25]. The pHpzc is the pH where the net surface charge is equal to zero. The surface charge of CA beads depends on the solution pH. Therefore, CA beads carry a positive charge below the pHpzc, but a negative charge above that. In our experiments with CA beads in 1 mM solution (Ex. 1a and b), where the solution pH was around 7.2, the surface of the CA beads would have been negatively charged, such that the electrostatic interaction between the CA beads and
LDHs have pHpzc of between 10 and 11[15]; therefore, they would have been positively charged under our experimental conditions (solution pH = 8.3). The negatively charged
Of the granular media used in our experiments, IHCS was most effective (86.0% in 1 mM solution) at microbial removal(Fig. 4). This could be attributed to the iron hydroxide (Fe(OH)3) coated on the exterior surface of QS having a positive surface charge (zeta potential = 9.7 mV), which effectively attracted the negatively-charged microbes. Note: the iron hydroxide was poorly crystallized, as indicated by the XRD data (Fig. 1b). Meanwhile, HCS was far less effective (35.6% in 1 mM solution) at microbial removal than IHCS (Fig. 4). This could be ascribed to the hematite (Fe2O3) coated on the external surface of QS being a crystallized iron oxide (Fig. 1c) with a negative surface charge(zeta potential = -1.8 mV).
The average percentage removals in 1 mM solution for both CA beads and LDH beads were higher than those in 10 mM solution (Fig. 5). This could be ascribed to the bacterial polysaccharides being fully extended from the cell surface at low ionic strengths, resulting in increased bacterial adhesion to the surfaces[29]. With the IHCS, the percentage removal remained constant on increasing the ionic strength from 1 to 100 mM (Fig.5). This might be attributable to
The microbial removals using LDHs and IHs immobilized on granular media were investigated using column experiments.The results showed that LDH beads were not effective for the removal of negatively-charged microbes, even though the positively-charged LDHs were contained in the beads. This could be related to the immobilization method, where LDH powders were immobilized inside the CA beads, with nano-sized pores; therefore, micro-sized microbes could not diffuse through the pores to come into contact with the LDHs in the bead, but would only adhere to the exterior surface of the bead via polymeric interaction. IHCS was most effective for the microbial removal, which could be attributed to the iron hydroxide coated on the exterior surface of the QS having a positive surface charge; therefore, would be effectively attracted to the negatively-charged microbes via electrostatic interaction. Meanwhile, HCS was far less effective than IHCS because the hematite coated on the external surface of QS is a crystallized iron oxide with a negative surface charge. This study has helped improve our knowledge on the potential application of functional granular media for microbial removal.