Soil microbes perform key functions in the nutrient cycles of forest ecosystems by effecting the decomposition of organic matter. The decomposition of plant litter on the forest floor is a critically important process for the integration of organic matter into humus. This is also relevant to the biogeochemical cycles of elements including carbon, nitrogen, phosphorus, and sulfur. Microorganisms can mineralize up to 80% of dead plant materials (Waring and Schlesinger 1986). As the importance of soil microbes has become increasingly recognized, many previous studies have been conducted in this regard ? including studies about the key variables controlling soil microbial activities. Temperature, pH, soil water content, land use changes, and nutrient availability have been identified as key variables controlling soil microbial activities (Kang and Lee 1998, Andersson and Nilsson 2001, Allison et al. 2008, Ye et al. 2009). According to our manipulation experiment, Q10 varied from 1.4 to 3.3 with different latitudes and types of soils (Winkler et al. 1996, Hopkins et al. 2006, Jin et al. 2008). Many studies have emphasized not only temperature but also soil water content as key controlling variables on microbial activities (Orchard and Cook 1983, Stark and Firestone 1995, Kang et al. 2003, Jia et al. 2006). Soil nutrients such as carbon, nitrogen, and phosphorous have also been identified as factors important to microbial activities (Wright and Reddy 2001, Jordan et al. 2003, Enowashu et al. 2009).
Microbial activity in soils can be estimated via respiration, arginine ammonification, or molecular tools such as stable isotope probing or enzyme activities (Paul and Clark 1989). Among these methods, enzyme activity analysis has been employed by a variety of researches. Enzyme activities are influenced by microbial community structures (Waldrop et al. 2000, Kourtev et al. 2002), biomass (Zak et al. 1994, Kennedy and Gewin 1997, Wellington et al. 2003) and physico-chemical properties of soil (Halvorson et al. 1996, Speir and Ross 2002). Therefore, enzyme activities are frequently utilized to evaluate the ecological integrity of soils, and as a general indicator of microbial activity (Killham and Staddon 2002).
The principal objectives of this study were: 1) to estimate soil enzyme activities of different types of extracellular enzyme activities in temperate forest soils, 2) to compare activities measured at different elevations, and 3) to determine key environmental factors for enzyme activities at different elevations.
This study was conducted on Mt. Jumbong (38º02' N, 128º26' E) in South Korea. Mt. Jumbong is a hardwood forest zone located at the southernmost outside area of the Mt. Seorak national park in Kangwon Province. The site is part of a UNESCO Biosphere Reserve and has been designated as a Natural Forest Reserve by the Korea Forest Service, Korea. The dominant fauna in this area are
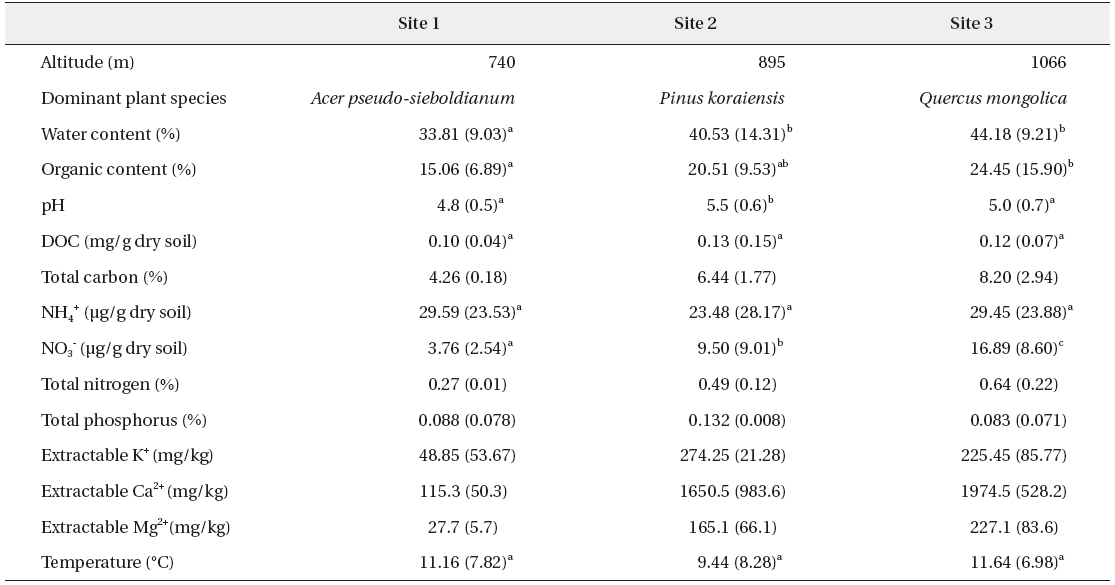
Spatial variations of average soil physico-chemical properties over 2 years (N: 34 for each site)
>
Soil physico-chemical analysis
Soil samples for chemical and enzyme analysis were collected from May, 2004 to September, 2005 on a monthly basis. Two replicate soils were collected at each site using a soil corer (5 cm dia. × 5 cm height) after removing the surface litter layer. Samples were transferred to a lab on ice, then sieved (2 mm) to remove large debris and stones.
Soil water contents were measured via oven-drying at 105℃ and the organic matter contents were determined via loss on ignition at 600℃. The pH levels were determined by a pH meter after mixing with distilled water and settling (soil:water = 1:2). DOC was extracted with distilled water and filtered through a 0.45 μm filter, and then analyzed via a TOC analyzer (TOC-VCPH; Shimadzu, Tokyo, Japan). Ammonium was determined via the indophenol blue method after extraction with 2 M KCl (Keeney and Nelson 1982). Nitrate was extracted with 0.5 M K2SO4 and measured by a colorimetric method (Anderson and Ingram 1993). Soil temperatures were measured with a soil thermometer. The data regarding precipitation and snow depth in this region were obtained from the Inje weather station, which is the nearest weather station.
>
Measurements of enzyme activities
Methylumbellifery (MUF)-substrates were employed as model substrates for soil enzymes. MUF-β-D-glucoside, MUF-N-acetylglucosaminide, MUF-phosphate and MUF-sulfate were used for β-glucosidase, N-acetylglucosaminidase, phosphatase, and arylsulfatase, respectively. 1.5 g soil samples were amended with 5 mL of substrate (400 μmol). After 60 minutes of incubation at 20℃, fluorescence in the supernatant was determined using a TD-700 fluorometer with a 450 nm emission and 330 nm excitation wavelength. For each sample, a calibration curve was prepared using 0-200 μmol of MUF-free acid, which was used due to its quenching effects and soil absorption properties (Kang and Freeman 1999).
To evaluate differences among the sites and sampling periods, data were analyzed via one-way ANOVA. Non-parametric correlation was employed to determine the relationship between environmental factors and methane oxidation rates. In an effort to model the relationship between methane oxidation rates and other environmental factors, we utilized a multiple linear regression model employing a stepwise regression algorithm. We conducted stepwise analysis with the average values of normalized enzyme activities and environmental factors. We normalized the glucosidase, N-acetylglucosaminidase and phosphatase by averaging them in accordance with the method of Kang and Freeman (2009). All analyses were conducted with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The results were considered to be statistically significantly different at
The pH of these soils varied between 4.8 and 5.5. Many of the soil properties in site 1 are different from those of sites 2 & 3. Although sites 2 & 3 were shown to differ in terms of their dominant vegetation, they evidenced similar chemical properties. Soil water contents, organic matter contents, and nitrate contents were significantly higher in sites 2 & 3 than in site 1 (Table 1).
>
Seasonal differences of enzyme activities
Mean values of β-glucosidase, N-acetylglucosaminidase, phosphatase, and arylsulfatases were 71.65, 22.59, 103.28, and 28.88 nmol g-1 dry soil min-1, respectively. These values were similar to those measured in other temperate forest soils (Harrison 1983, Speir and Cowling 1991, Trasar-Cepeda et al. 2000) and were slightly higher than in cold areas (Haussling and Marschner 1989). β-glucosidase and phosphatase evidenced higher levels of activity than N-acetylglucosaminidase and arylsulfatase. We noted no significant seasonal variations in β-glucosidase, N-acetylglucosaminidase, or phosphatase; those enzyme activities evidenced similar values throughout the entire year, even during the winter. However, arylsulfatase evidenced a marked seasonality pattern, with much higher values in summer than in winter (data not shown). This is consistent with the results of a previous study (Kang and Freeman 1999). Acosta-Martinez et al. (2007) also noted that arylsulfatase exhibits a different origin from other enzymes, and may be controlled in a different manner. Other enzymes may be influenced by other factors such as the organic matter supply (Vance and Chapin 2001) and water availability. As a consequence, those enzyme activities may not evidence peak activity levels over the summer (Bonnett et al. 2006). Further, the method utilized in this study was conducted using saturated concentrations of substrates and optimal temperatures. As such, this method provided potential activities rather than actual activities, that may be influenced profoundly by temperature and edaphic conditions.
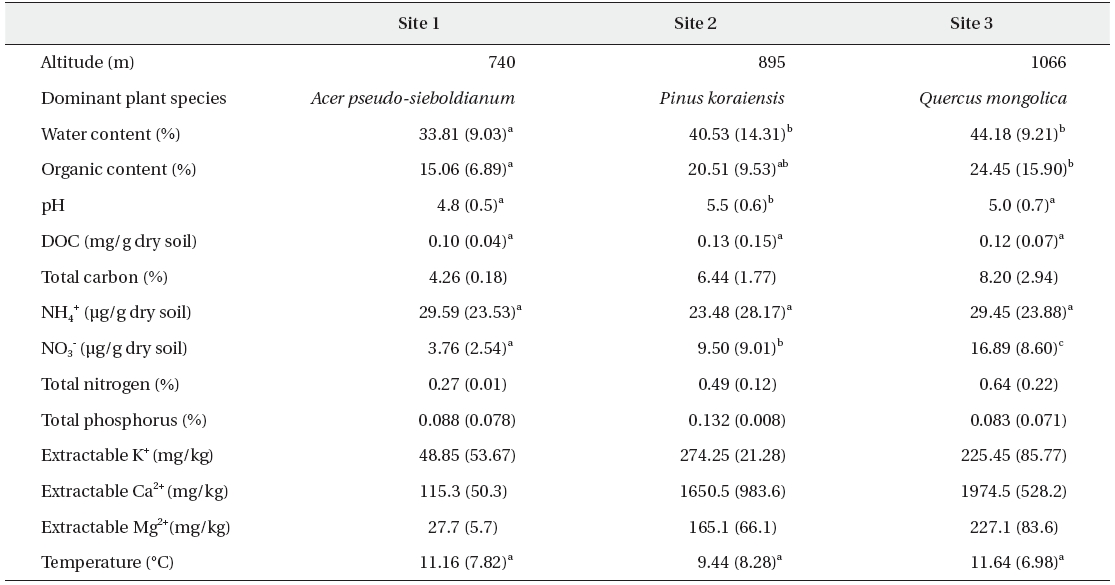
Interestingly, the ratio between different enzymes is consistent over seasons and sites. Fig. 1 shows a similar ratio between β-glucosidase and N-acetylglucosaminidase in different sites and on different months. This is consistent with a report from Sinsabaugh et al. (2009) who noted similar scaling of C, N, P acquisition enzymes across a variety of ecosystems. Although substantial variations of enzyme activities were noted on a small scale, the overall enzyme ratios may remain constant.
>
Spatial differences of enzyme activities
The enzyme activities in sites 2 & 3 were significantly higher than those of site 1 for every enzyme. In particular, β-glucosidase and N-acetylglucosaminidase clearly evidenced these trends (Fig. 1). Additionally, phosphatase levels were significantly different at every site (data not shown). For every enzyme activity, the values in site 3 are higher than those in site 1.
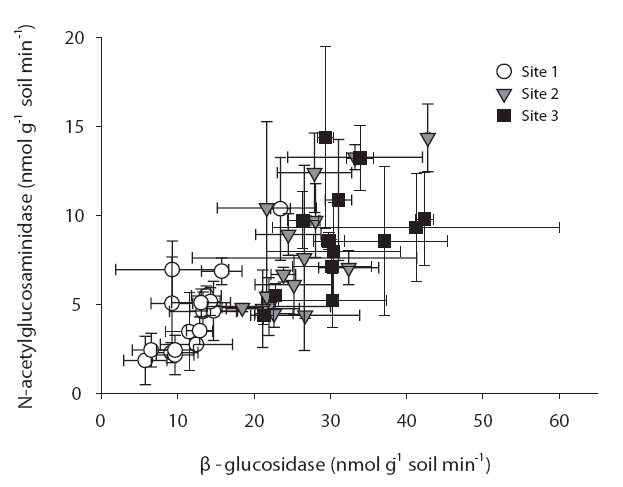
We conducted correlation analysis separately for each site. Because there were no significant seasonal differences, we composited the data and analyzed it with different sites, and thus the numbers of data collected were 34 and 62 for site 1 and sites 2 & 3, respectively. The β-glucosidase, N-acetylglucosaminidase, and phosphatase activities measured at site 1 were significantly correlated with organic matter contents (r = 0.467,
Enzyme activities = 0.013 × Temperature + 0.12 × Organic matter content + 0.326
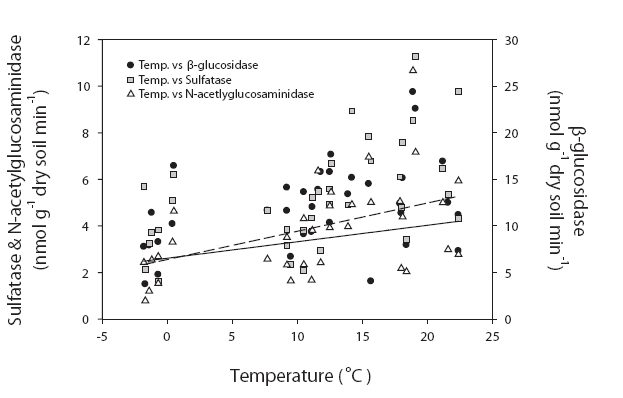
On the other hand, the values at sites 2 & 3 were significantly correlated with water content (
Enzyme activities = 25.864 × NO3- + 0.014 × water content + 5.701 × NH4+ + 0.140
On the other hand, the values at sites 2 & 3 were significantly correlated with water content (r = 0.675, 0.591, and 0.406; P < 0.001), organic matter contents (r = 0.599, 0.481, 0.349, and 0.384; P < 0.05) and inorganic nitrogen ion content (r = 0.343-0.548, P < 0.05) (Fig. 3). The following regression equation explained 56% of the variations in average enzyme activities.
Enzyme activities = 25.864 × NO3- + 0.014 × water content + 5.701 × NH4+ + 0.140
Enzyme activities differed significantly among the sites in our study. These differences were particularly distinctive between site 1 and sites 2 & 3. Importantly, the dominant tree species of these locations were different. The dominant species in sites 1 and 3 were deciduous forest, and conifers were dominant at site 2. Despite this difference in tree species, the enzyme activities differed between site 1 and sites 2 & 3. Therefore, the soil properties appear to be more salient than the predominating tree species. This was consistent with the conclusions of Ushio et al. (in press). They concluded that soil physicochemical properties were critically important to enzyme activities. Although the tree species affect the physicochemical properties of soil, the properties of soil may have a more direct effect on enzyme activities. The enzyme activities evidenced significantly higher values in sites 2 & 3 than in site 1. The results of regression analyses also demonstrated different controlling variables between site 1 and sites 2 & 3. The physico-chemical properties of soil, such as organic matter contents, exchangeable cation, nitrate, and ammonium ion contents were higher at higher elevations. It has been suggested that enzyme activities in site 1, which is characterized by low nutrient availability and organic matter contents, are strongly controlled by temperature (Fig. 2). By way of contrast, enzyme activities in fertile soils in sites 2 & 3 are more profoundly influenced by nitrogen availability (Fig. 3). Enzyme activities have been reported to be strongly affected by microclimates in degraded soils. For example, Boerner et al. (2005) reported that different types of enzymes evidenced different relationships with abiotic factors in degraded soils, such as thinning and fired soils. For example, chitinase and acid phosphatase contents were regulated by microclimate, whereas lignocellulose-degrading enzymes were affected most prominently by substrate availability. The overall results of this study show that soil fertility characteristics may impose controls on soil enzyme activities and influence key controlling variables.