According to Valle et al. (1985), the leaf temperature of soybeans was approximately 1.5℃ higher at elevated CO2 concentrations, and leaf resistance was more profound at elevated CO2 concentrations than under ambient CO2 conditions. As a consequence, the transpiration rate of soybeans grown under ambient or elevated CO2 concentrations was not significantly affected by alterations in CO2 levels. Because the increases in leaf resistance caused by high CO2 were partially offset by increases in the leaf-to-air vapor pressure gradient induced by the increased transpiration rate owing to increased leaf temperature (Polley et al. 2008). In other words, higher temperatures can increase the transpiration rate by altering the vapor pressure deficit at the leaf surface. From the above discussion, the transpiration rates of P. insularis and P. americana would be more profoundly affected by increased temperature than by elevated CO2 concentrations.
Is has been theorized that, because atmospheric CO2 concentration and air temperature appear to be linked, and elevations are occurring in both, the global environment should also be experiencing a concomitant change (Morison and Lawlor 1999).
Since the late 1950s, global atmospheric CO2 concentration has increased by an average of 1.9 ppm per year (Intergovernmental Panel on Climate Change 2007). Within this century, atmospheric CO2 concentration is expected to exceed 50 ppm (Hofmann et al. 2009), in turn generating a global mean surface temperature warming of 1.9-4.4℃ (Intergovernmental Panel on Climate C5hange 2007). However, according to Lunt et al. (2010), the earth's temperature might be as much as 30- 50% more sensitive to atmospheric CO2 concentration than previously thought.
In Korea, since the late 1990s, atmospheric CO2 concentrations rose from 370.7 ppm to 391.4 ppm in 2008 (Korea Meteorological Administration 2008). According to the observational data from six observation stations (in Seoul, Incheon, Gangneung, Daegu, Mokpo, and Busan) between 1912 and 2009, the annual mean temperature has risen by 1.7℃ in that time (Korea Meteorological Administration 2009).
First, most of the plants increase the photosynthetic rate under elevated CO2 and temperature (He et al. 2005, Geissler et al. 2009). Additionally, a common response of plants to elevated CO2 concentration is reduced stomatal conductance (Leakey et al. 2009a). This frequently results in a reduced transpiration rate, coupled to a resultant increase in the plant's water use efficiency (Radoglou et al. 1992, Kanemoto et al. 2009).
Second, elevated CO2 and temperature conditions tend to alter the foliar chemistry of plants. Generally speaking, the chlorophyll contents of leaves grown under elevated CO2 concentrations are reduced (Wullschleger et al. 1992, Hamid et al. 2009). Additionally, leaf nitrogen contents are reduced, but carbon contents are increased under such conditions (Gifford et al. 2000). Consequently, the change in the relative proportion of carbon to nitrogen is increased substantially under elevated CO2 and temperature conditions (Rao et al. 2009).
These physiological responses of plants to elevated CO2 and temperature are known to vary due to interspecific differences or varying experimental conditions, as well as for several other reasons (Enoch and Honour 1993, Hamilton et al. 2008). In foreign countries, many researchers have measured plants' physiological responses to global warming using a variety of techniques, methods and plant materials, as well as interactions among different environmental factors. While in Korea, the influence of elevated CO2 or temperature on physiological responses of crops to enhance crop productivity has been studied (Lee and Choi 2001, Lee et al. 2009), but the effect of global warming on physiological responses of native plants have never been studied so far.
Native plants are generally less tolerant to environmental stresses and have lower phenotypic plasticity in acclimating to a broader range of environmental conditions than invasive species (Sakai et al. 2001, Baruch and Jackson 2005). For the reasons mentioned above, native species may be more negatively affected than invasive species under the global warming. According to Song et al. (2009), the photosynthesis and biomass production of native species under elevated CO2 were lower than their invasive competitors.
In order to compare the physiological responses of native and invasive plants to elevated CO2 and temperature, we measured the photosynthetic parameters, chlorophyll contents, nitrogen contents, carbon contents and C/N ratios of
A native species restrictively found in Ulleung-do of Korea,
>
Experimental design and growth condition
This study was conducted in and out of a glass greenhouse. The control was maintained at ambient CO2 concentration and temperature (AC-AT) of the immediately surrounding air, which averaged approximately 370-380 ppm on a 24-hour basis. In order to ensure the same light intensity in the treatment, the controls were covered by the glass roof as well.
This study was conducted in and out of a glass greenhouse. The control was maintained at ambient CO2 concentration and temperature (AC-AT) of the immediately surrounding air, which averaged approximately 370-380 ppm on a 24-hour basis. In order to ensure the same light intensity in the treatment, the controls were covered by the glass roof as well.
In this fashion, the elevated CO2 concentration was maintained at approximately twice that of the ambient (750-800 ppm). An LCi Ultra Compact Photosynthesis System (Lci Pro; ADC Bioscientific, Hoddesdon, UK) was used to evaluate the stability of the CO2 concentration in the treatment. The CO2 concentration was controlled from the planting throughout the experiment.
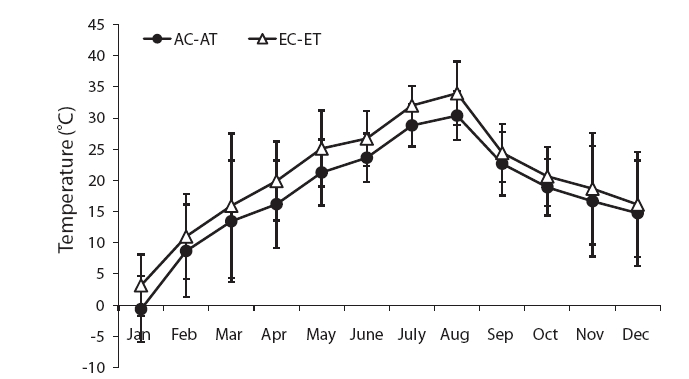
The mean temperature in the treatment was about 3℃ higher than the control (Fig. 1). The air temperature was measured using an alcohol thermometer at the same height in the control and treatment during the study period.
In November 2007, the matured seeds of two species were collected from several individuals of a population in a glass greenhouse, respectively. In May 2008, the seeds of two species were sown in pots (51 cm × 15.3 cm × 12 cm) filled with equal proportions of sand, and we fertilized 0.5% of the sand weight. We subsequently applied organic fertilizer, which contains an ammonium nitrogen content of below 170 mg/L and nitrate nitrogen at a concentration of 150-330 mg/L. The plants were watered twice or three times per week to prevent them from suffering from water stress.
In June 2008, the plants were grown in the glass greenhouse until reaching the 2 to 3-leaf growth stage and were then transplanted in 2-seedlings into pots (22.5 cm × 27 cm) containing the sand.
For each species, three replicate pots were randomly assigned to the control and treatment respectively.
The photosynthetic characteristics of the two species were measured at the vegetative stage, which was developmentally determined for the control and treatment using an LCi Ultra Compact Photosynthesis System (Lci Pro) from 10:00 am to 12:00 pm in June, 2009.
Leaf sections for measurements were selected in the upper parts of fully expanded leaves per an individual plant. The measured leaves from the controls were initially exposed to an air CO2/ concentration of 370-380 ppm and those from the treatment to a CO2concentration of 750-800 ppm.
Twenty four hours before measuring photosynthesis, water was supplied to the level of the moisture capacity (700 mL) of a pot, in order to reduce the difference in relative humidity between the control and treatment. The light source utilized for the natural light and photosynthetic active radiation was 400-600 μmol m-2s-1 in our measurements. Before the air entered the leaf chamber, the LCi Ultra Compact Photosynthesis System removed water vapor in the air through columns of soda lime.
All measurements were replicated more than 30 times. The items measured included the photosynthetic rate (μmol m-2s-1), stomatal conductance (mol m-2s-1), transpiration rate (mmol m-2s-1) and water use efficiency (μmol CO2/mmol H2O).
>
Chlorophyll content measurements
Nitrogen content measurements
The percentage change of the measured physiological parameters of two species relative to the controls was represented via the method of Ghannoum et al. (2010).
>
Photosynthetic characteristics
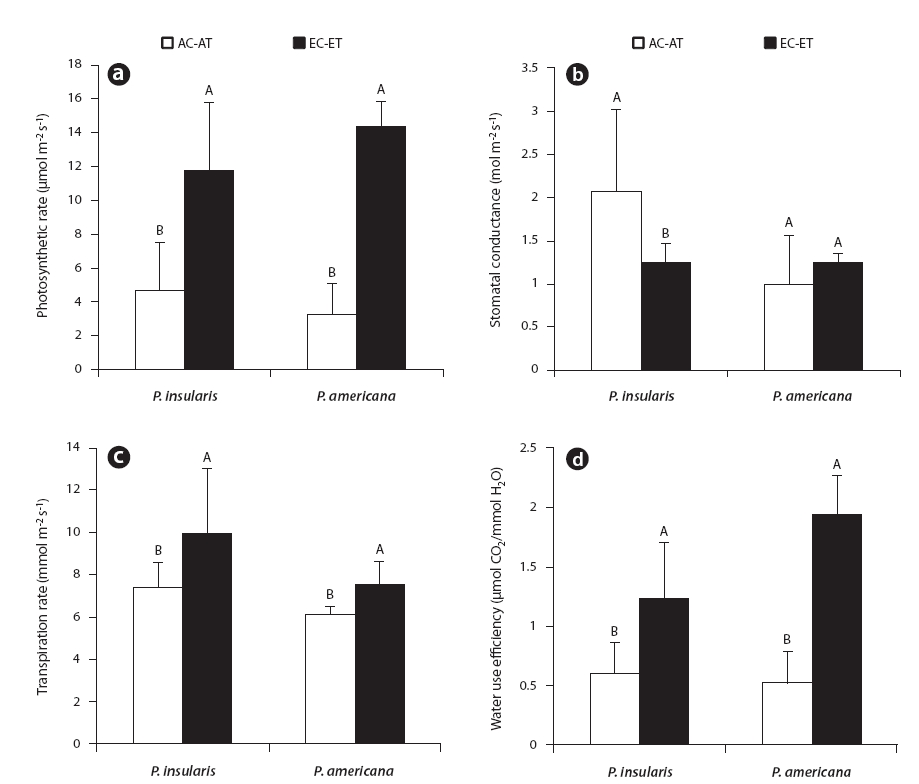
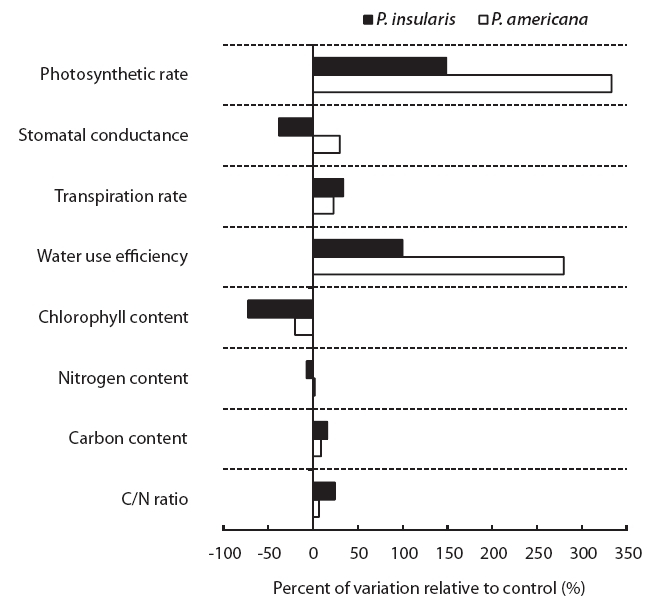
The photosynthesis rate (Fig. 2a), transpiration rate (Fig. 2c) and water use efficiency (Fig. 2d) of the two plant species were higher under the treatment than under the control. Among the photosynthetic characteristics, photosynthesis rate and water use efficiency were found to be particularly related to plant growth. The photosynthesis rate of
At treatment, the stomatal conductance (Fig. 2b) of
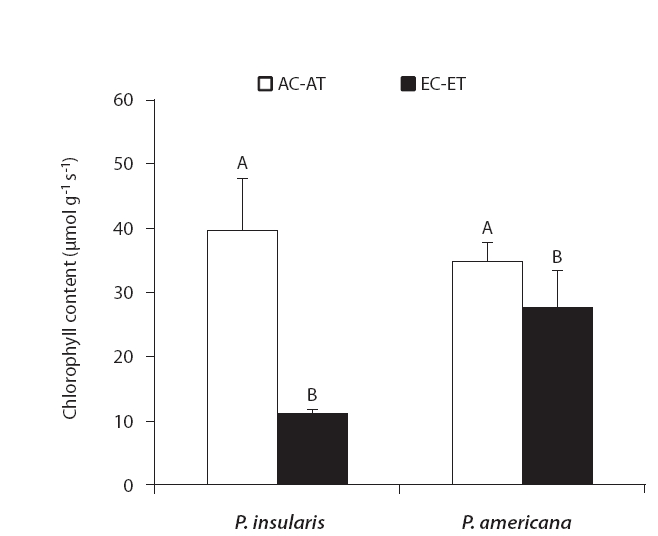
The results of chlorophyll content of two plant species on elevated CO2 and temperature are shown in Fig. 3. Chlorophyll content of two species significantly decreased by elevated CO2 and temperature.
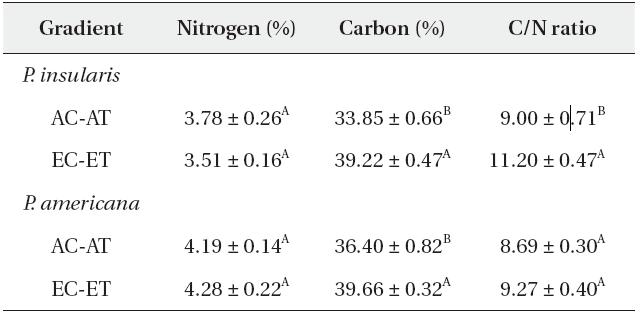
The nitrogen contents of two plant species were not affected significantly by elevated CO2 and temperature (Table 1), whereas the carbon contents of
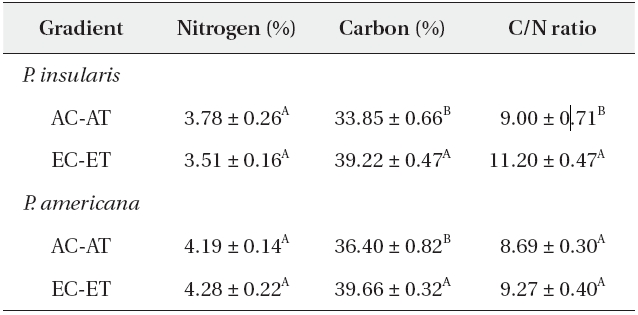
Nitrogen content, carbon content and C/N ratio of Phytolacca insularis and Phytolacca americana grown in control (AC-AT) and treatment(EC-ET) conditions
When we compared the two plant species grown under control and treatment conditions, the physiological responses of the two plant species grown under control and treatment conditions were different (Fig. 4).
In general, elevated CO2and temperature enhance the photosynthetic rates of leaves (Lemon 1983, Lee and Choi 2001, Ghannoum et al. 2010) and water use efficiency (Nijs et al. 1989), and reduce the stomatal conductance and transpiration rates of the plants (Morison 1987, Ainsworth and Rogers 2007).
The results of our measurements demonstrated that the photosynthetic rates of
Several previous studies have suggested that a reduction in photosynthesis was directly caused by excessive starch accumulation (Onoda et al. 2007, Leakey et al. 2009b), declines in chlorophyll content (Croonenborghs et al. 2009), reductions in Rubisco activity and RuBP regeneration (Zhang et al. 2008, Yamori and von Caemmerer 2009), etc.
We determined that the chlorophyll contents of
This means that plants grown under elevated CO2 concentration conditions tend to be more efficient in the capture or use of irradiance for photosynthesis than plants grown at ambient CO2 levels. Thus, it is reasonable to suppose that increases in the photosynthesis of
In most plants, stomatal conductance declined with increases in the levels of CO2, because stomata were closed more often at high CO2 concentrations (Bazzaz 1990). As a consequence, transpiration rates were reduced with consequent increases in water use efficiency (Bazzaz 1990). The stomatal conductance of
Lee et al. (2001) previously reported a 23% reduction in the stomatal conductance of 13 perennial species grown for 2 years under elevated CO2 levels. The endangered Western Himalayan herb,
Elevated CO2 concentrations frequently reduce the stomatal conductance of plants and also may reduce the transpiration rate. However, elevated CO2 concentration and temperature may not always reduce the transpiration rate (Dugas et al. 1997, Zheng et al. 2010), because of other compensatory effects occurring under elevated CO2 and temperature conditions, such as increased leaf temperature--which might cause increased leaf-air vapor pressure deficits (Katul et al. 2009).
In our study, the transpiration rates of
According to Valle et al. (1985), the leaf temperature of soybeans was approximately 1.5℃ higher at elevated CO2 concentrations, and leaf resistance was more profound at elevated CO2 concentrations than under ambient CO2 conditions. As a consequence, the transpiration rate of soybeans grown under ambient or elevated CO2 concentrations was not significantly affected by alterations in CO2 levels. Because the increases in leaf resistance caused by high CO2 were partially offset by increases in the leaf-to-air vapor pressure gradient induced by the increased transpiration rate owing to increased leaf temperature (Polley et al. 2008). In other words, higher temperatures can increase the transpiration rate by altering the vapor pressure deficit at the leaf surface. From the above discussion, the transpiration rates of
Increasing CO2 concentration may generally have the effect of enhancing the water use efficiency of plants, because partial stomatal closure reduces transpiration (Ainsworth and Rogers 2007).
According to our results, the water use efficiency of
Invasive species generally evidence higher photosynthetic rates and greater water use efficiency than native species (McAlpine et al. 2008). Pattison et al. (1998) compared five invasive plants and four native plants, and determined that all invasive species evidenced higher photosynthetic rates than the native plants. Deng et al. (2004) also determined that the invasive plant,
In EC-ET, photosynthetic rate and water use efficiency of
Enhanced CO2 concentration associated with increasing temperature was predicted to effect alterations in the biochemical components of plant tissues, including nitrogen contents, carbon contents, and C/N ratios (Cotrufo et al. 1998, Gifford et al. 2000, Zhou and Shangguan 2009).
Generally, the carbon contents increased but the nitrogen contents were reduced in the leaves, and resulted in higher C/N ratios under elevated CO2 concentrations. A meta-analysis of the 75 reports of the effects of elevated CO2 on herbivores and host plants determined that the nitrogen contents of plants were reduced by 16.4%, whereas the C/N ratios were increased by 26.6% (Stiling and Cornelissen 2007). Contrary to the prevalent view, the nitrogen contents of
The C/N ratio of
According to our results, the C/N ratio of
In conclusion, our results demonstrated that physiological responses of