Allelopathy is defined as any direct or indirect harmful effect of a plant on another organism (including microorganisms) through production of chemical (Rice 1984). Secondary metabolites may function in plant defense via allelopathic processes (Fernandez et al. 2009).
In recent years, there has been increasing interest in healthy lifestyles and healthy aging. As a result, many people are involved in searches for natural compounds that can improve health, especially those of plant origins. A great number of aromatic, spicy, medicinal, and other plants belonging to the family Asteraceae contain chemical compounds exhibiting antimicrobial and antioxidant properties (Boussaada et al. 2008). Antimicrobial and antioxidative plant oils and extracts have been used for many purposes, including raw and processed food preservation, pharmaceuticals, alternative medicines, and natural therapies (Hammer et al. 1999). Natural products are perceived as having fewer negative impacts than synthetic agents; natural products may be effective, selective, biodegradable, and less toxic to the environment. The genus
The aerial parts of A.
The test microorganisms included three gram-positive bacteria (
>
Extract preparation for antimicrobial activity of two Artemisia plants
We soaked 200 g samples of air-dried leaves of
The crude methanol extract was partitioned with 500 mL of hexane and then the top layer was concentrated (comprising the hexane fraction). The remaining layer was successively fractionated with 500 mL of diethyl ether and then ethyl acetate (forming the ether and ethyl acetate fractions). The remaining residue was the water fraction. Each fraction was concentrated
>
Determination of antimicrobial activity
Each bacterial strain was grown in a nutrient broth at 30℃ for 18-24 hours prior to testing and subcultured three times for another 18-24 hours. The turbidity of bacterial cell suspensions was brought to 0.3 optimal density (OD) at 660 nm by adding sterile broth and was then used for the tests. We poured 0.1 mL of the bacterial cell suspensions uniformly on nutrient broth agar plates. The paper disks containing the extracts (ether fraction and ethyl acetate fraction) were carefully placed on the seeded Petri dishes. The diameters of the resulting inhibition zones were measured in mm after the cultures were incubated at 30℃ for 24 hours or 48 hours (Bauer et al. 1966). The antimicrobial activity was calculated as the net zone of inhibition estimated from the growth inhibition zone measurements (Mahasneh and El-Oqlah 1999). The minimum inhibitory concentration (MIC) was determined as the lowest concentration that caused an inhibition zone. We measured the inhibition caused by 0.1, 0.25, 0.5, 1.0, 1.5, 2.0, and 3.0 mg/mL extracts.
>
Gas chromatography-mass spectrometry (GC-MS)
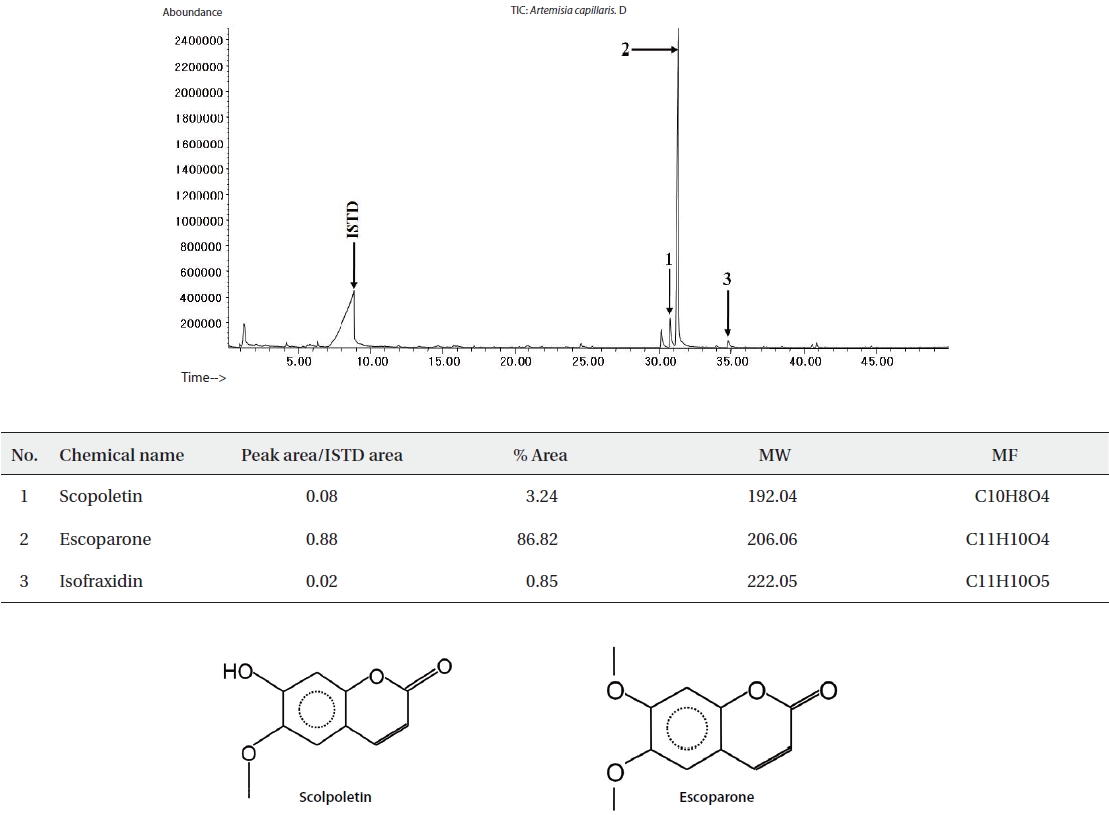
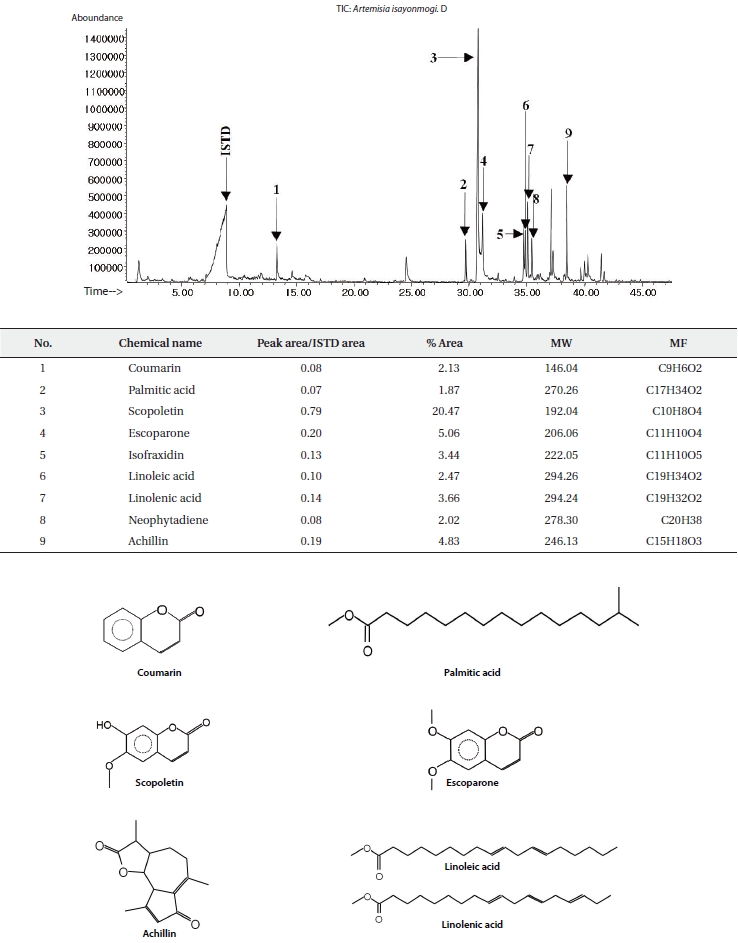
The ethyl acetate fractions of
We used a randomized complete block design with four replications in all experiments. Each experiment was repeated three or four times. Statistical analysis was conducted using the software program SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). Comparisons between treatments were made at the 0.05 level using Duncan’s multiple-range test.
>
Antimicrobial activity of the two Artemisia plants
Local people use different plant parts to prepare phytomedicines, and the most frequently used parts are aerial parts, including leaves, fruits, seeds, and flowers (Kultur 2007). The antimicrobial activity and MIC of the ether and ethyl acetate fractions of methanol extract from the two
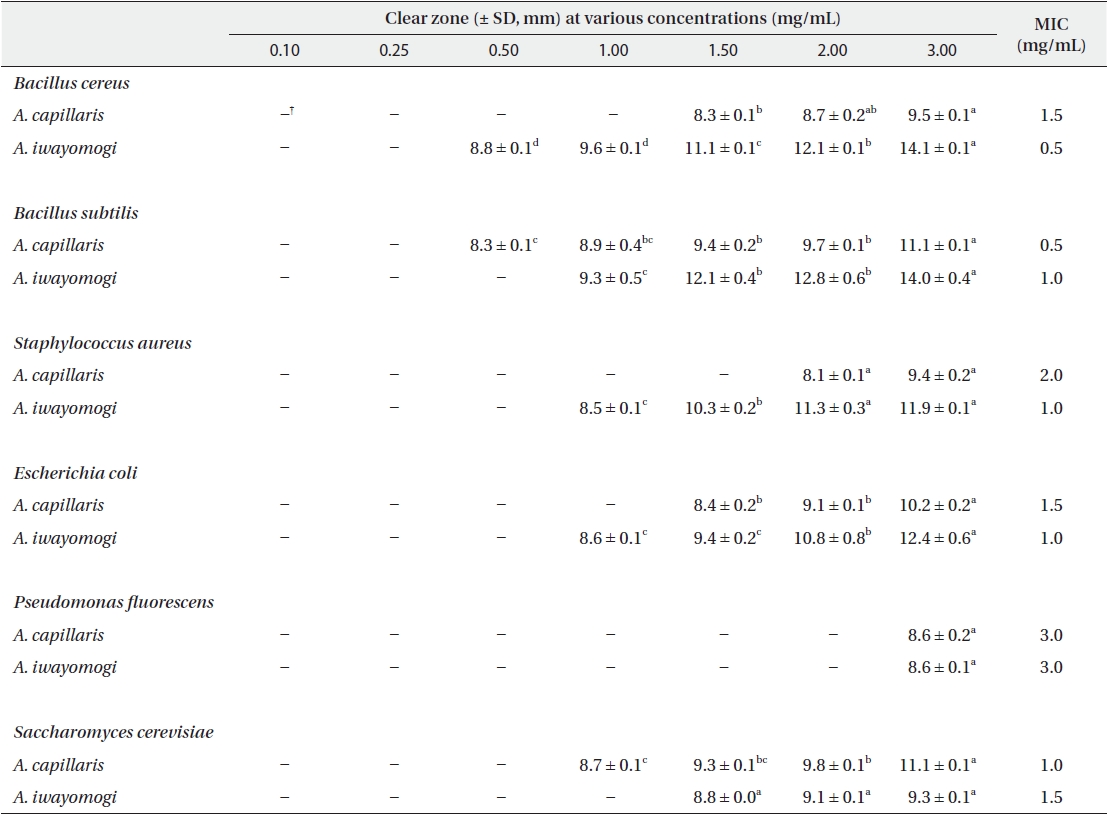
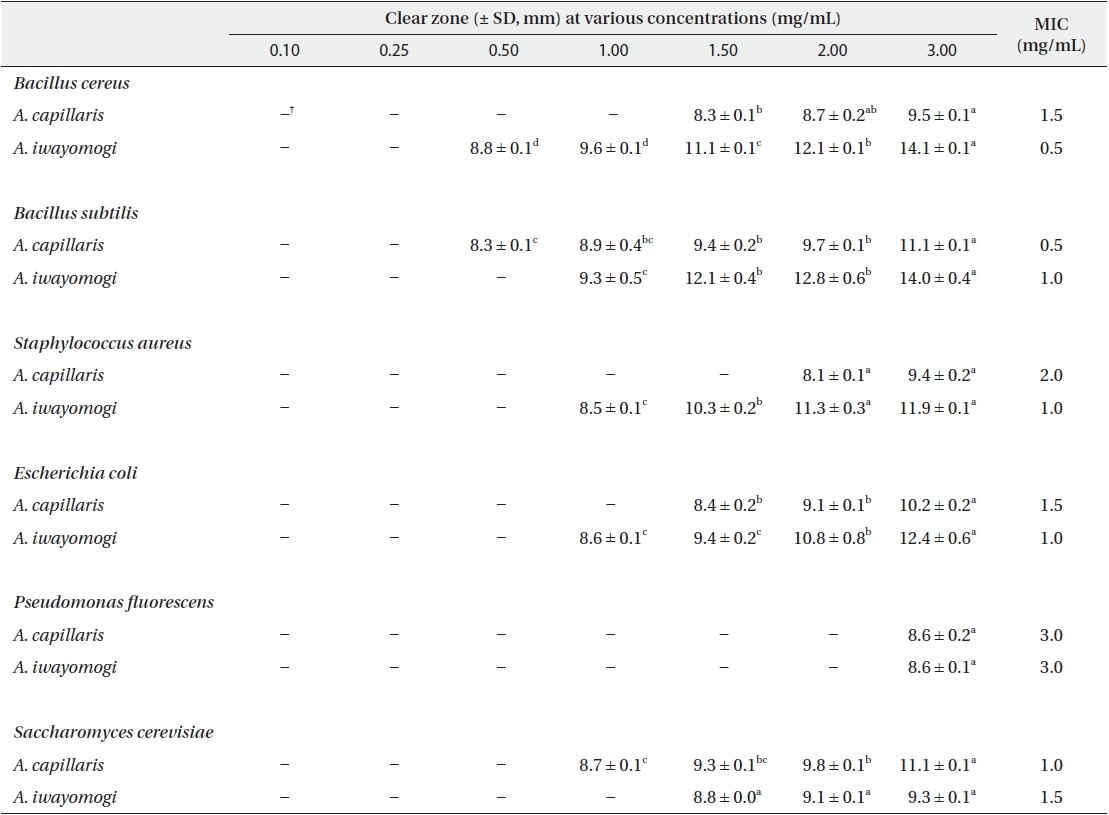
Antimicrobial activity of the ether fraction of methanol extracts from Artemisia capillaris and Artemisia iwayomogi*
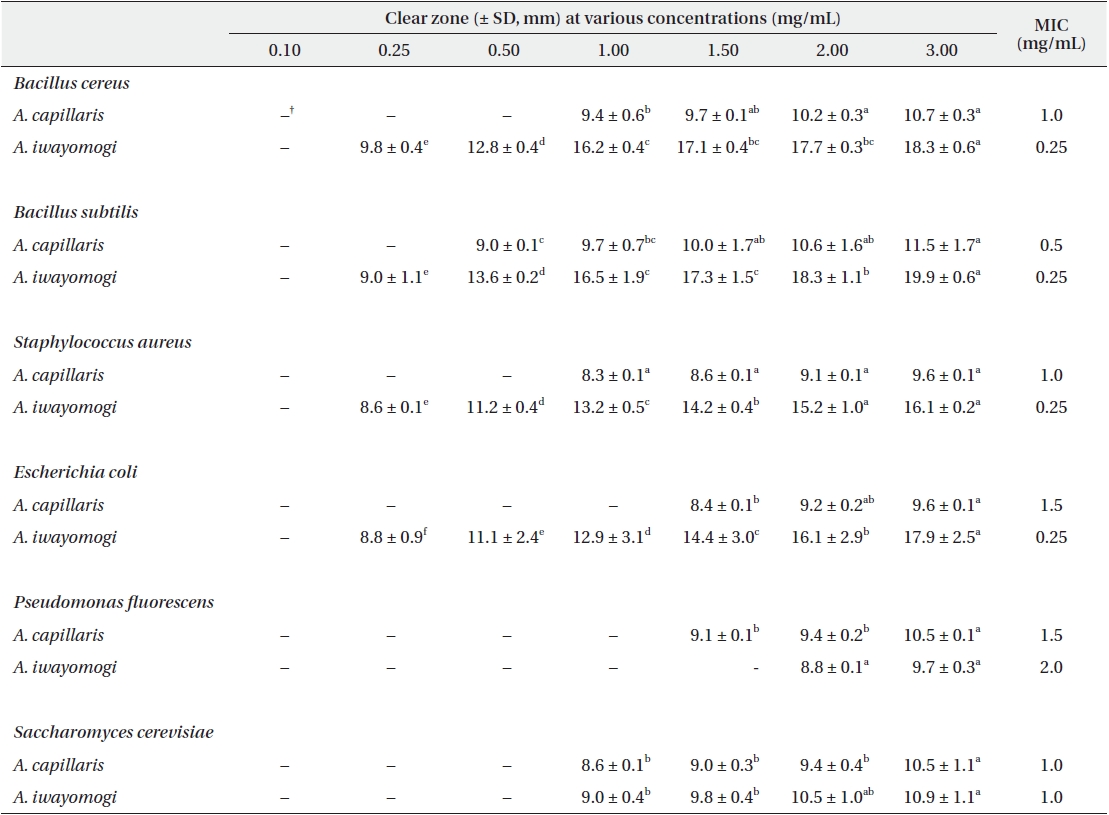
Some gram-negative bacteria are less sensitive than other microbes to the action of plant extracts and compounds (Boussaada et al. 2008, Yun et al. 2008), but gram-negative bacteria are often more susceptible than gram-positive bacteria to the inhibitory effects of essential oils (Smith-Palmer et al. 1998). In our study, the ether and ethyl acetate fractions of the plant extracts were more active against gram-positive bacteria than gram-negative bacteria and yeast: the antimicrobial action of the ethyl acetate fraction of
The ethyl acetate fraction of extracts of
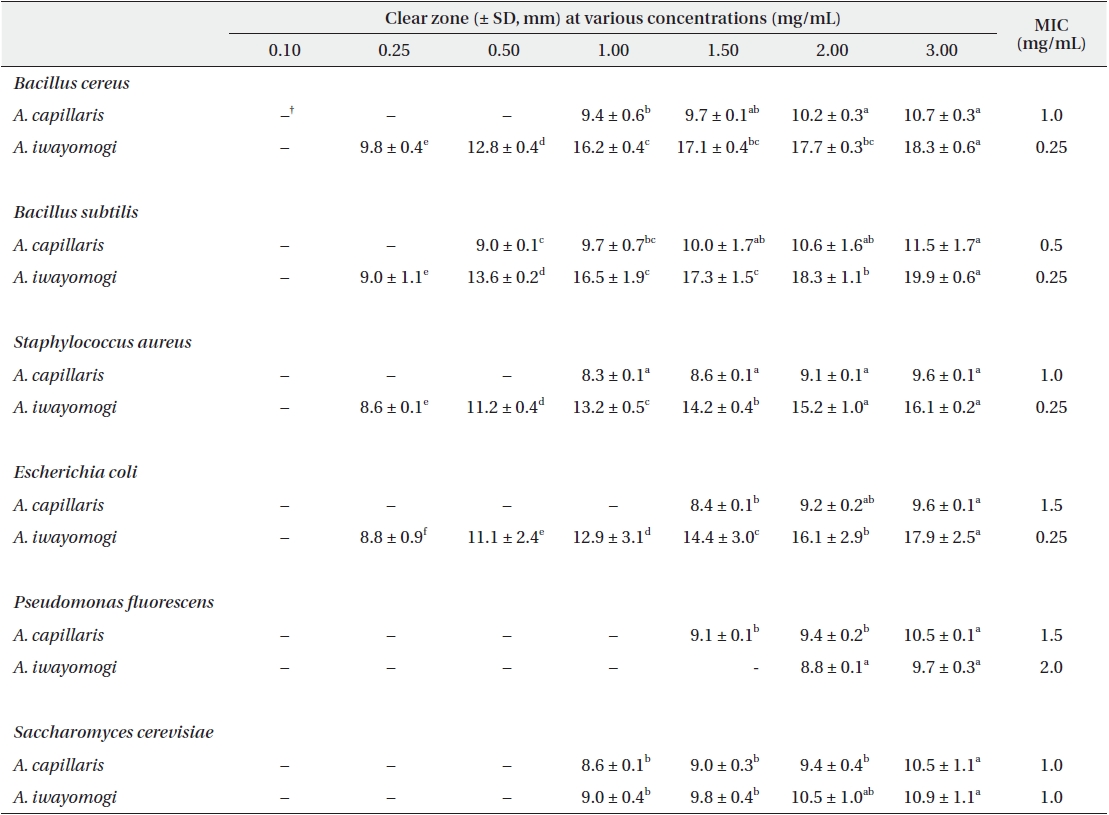
Antimicrobial activity of the ethyl acetate fraction of methanol extract from Artemisia capillaris and Artemisia iwayomogi*
Most phenolics that display antimicrobial activity are phenolic acids or flavonoids. Phenolic acids are a major class of phenolic compounds occurring in a diverse range of plants (Wojdyło et al. 2007), and the phenolic moiety plays an important role in determining a plant’s antimicrobialactivity (Kujumgiev et al. 1993). And mixtures of phenolic acids and other organic compounds can cause inhibitory effects even when the concentrations of the individual compounds are well below inhibitory levels (Blum 1996).
Our results provide the first detailed documentation of the