At their current rate of increase, CO2 levels are expected to double to 750 ppm by the end of this century. Elevated CO2 significantly influences both soil nutrient availability and soil microbes that are associated plants (Janus et al. 2005). Numerous studies that have investigated the effects of elevated CO2 on plants found that elevated CO2 increases the growth rate of plants (Gifford 1994, Naidu et al. 1998) while significantly affecting physiological function (Melillo et al. 1990). In addition, elevated atmospheric CO2 causes an increase in the C:N ratios of plants by reducing the N concentration (Berntson and Bazzaz 1996). Such results were due to changes in the level of Rubisco or respiratory proteins, or to the dilution of nitrogen resulting from the accumulation of non-structural carbohydrates. Sims et al. (1998) recently reported that nitrogen levels are higher in plants under elevated CO2. However, Saxe et al. (1998) found that elevated CO2 had no effect on the nitrogen or carbon content of oak and beech seedlings. These results suggest the level of nitrogen in plant tissue exposed to elevated CO2 is species-dependent.
Enzymatic changes under elevated CO2 can alter the microbial demand for N and therefore the flow of N between soil microorganisms and plant roots (Zak et al. 2000). This in turn alters the overall chemical composition of plants as well as the types of organic substrates available for microbial metabolism (Finzi et al. 2006). Elevated CO2 can also increase microbial activity in the soil (Hungate et al. 1996) mainly by providing extra C sources for rhizospheric microorganisms (Zak et al. 2000). Soil microbial activity can be measured in terms of CO2 production or based on the activities of major metabolic enzymes (Klose et al. 2003).
The responses of plants and microorganisms to elevated CO2 vary according to the plant species (Cotrufo et al. 1998). While much information is available on the effect of elevated CO2 on trees, little is known about how pine seedlings are affected. Since pine trees are a dominant tree species in eastern Asia, we believe that such information would be highly valuable.
The purpose of this study was to investigate the growth of pine seedling as well as soil microbial activity in response to elevated CO2 in a growth chamber. We report on the impact of increased CO2 on the root and shoot growth, biomass (dry weight) and C:N ratio of Pinus densiflora, emphasizing the interdependency of soil chemistry and microbiology, soil moisture and plant growth.
Natural soil was sampled from the pine forest on the Ewha Woman’s University campus in Seoul, Korea. Soil samples (1 kg/pot, diameter 10 cm) were used for the planting of three-year-old pine seedlings (
>
Pine seedling growth and C:N ratio analysis
The pine seedlings were planted in a growth chamber at 25°C and 60% humidity, and were subjected to a 16 h light/8 h dark cycle. Shoot, root length, and biomass (dry-weight) were measured every four months. All tests were performed in triplicate. Percent dry weight of N and C content were estimated from leaf and root powder using a Flash EA 1112 Analyzer (Thermo Electron Corporation, Waltham, MA, USA).
Soil pH was determined by adding soil to water at a ratio of 1:5 (w:v). Soil moisture was determined gravimetrically by drying at 105°C for 24 hours, and organic matter content was determined by loss on ignition at 700°C (MAS 7000 oven; CEM, Mattews, NC, USA). Soil cation-exchange capacity was determined according to EPA 9081 methods (US Environmental Protection Agency 1986). Soil nitrate (NO3-) content was determined by extracting soil with deionized water and then measuring NO3- content in the liquid phase using an NO3- electrode (Gelderman and Beegle 1998).
>
Analysis of dissolved organic carbon and phenolic compounds in soil
To measure the concentration of DOC, soil was added to water at a ratio of 1:10 (w/v) after which the DOC content was measured using a TOC 5000 (Shimadzu Co., Kyoto, Japan) meter. Specific UV absorbance (SUVA) reveals the nature or quality of DOC in a given sample and is used as a surrogate measurement of DOC aromaticity (Chin et al. 1994). SUVA was measured at 254 nm (SUVA254) due to the strong absorption of natural organic matter at this wavelength. This value correlates strongly with the aromatic carbon content of organic matter (Chin et al. 1994). Phenolic compound content was assayed using Folin-Ciocalteau phenol reagent (Box 1983). One milliliter of sample was added to 1.5 mL of Na2CO3 solution (50 g/L). Then, 0.5 mL of Folin-Ciocalteau solution (diluted 1/4 with deionized water; 0.5 N) was added, followed by incubation of the mixture for 2 hours at room temperature. A standard curve was prepared by applying the same chemicals to a series of 0 to 2 mg/L phenol solutions.The color change of reactants was measured spectrophotometricallyat 750 nm. Once out of range of the standard curve, the samples were diluted with distilled water and the procedure was repeated. Phenol oxidase activity was determined using 10 mM L-dihydroxyphenylalaninesolution as a substrate, according to Pind et al. (1994).
>
Analysis of microbial activity in soil
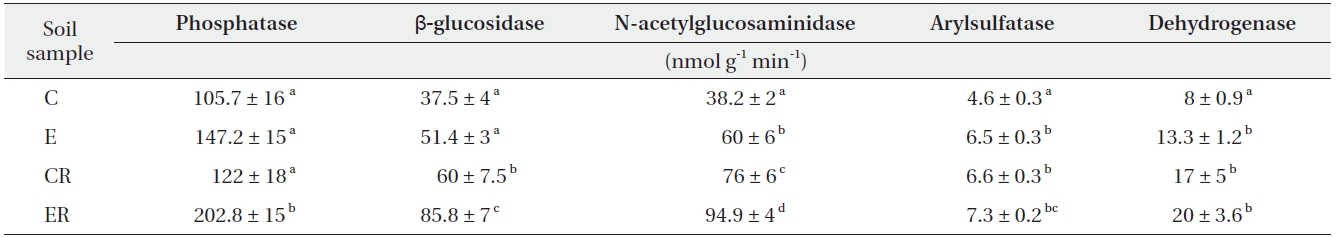
The activities of four extracellular enzymes (β-glucosid ase, N-acetylglucosaminidase, phosphatase, and arylsulfatase) were measured by the MUF-substrate method (Freeman et al. 1996). The concentrations of the MUF-β-glucoside, MUF-N-acetylglucosamine, and MUF-arylsulfate substrate solutions were 400 μM (Sigma, St. Louis, MO, USA) while the concentration of the MUF-phosphate substrate solution was 800 μM (Sigma). Enzyme activities in a slurry containing soil and substrate solution (1:5 w/v) were measured using a fluorimeter. Dehydrogenase activity was measured by 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (INT) assay (Tabatabai 1982). Mixtures of soil (3 g fresh soil) and substrate solution were incubated for 24 hours at 37℃ after which the reaction products were detected using a spectrophotometer (DR/3000 Spectrophotometer; HACH, Mount Holly, NJ, USA) at 485 nm.
Data were analyzed by one-way ANOVA using SPSS ver. 9.0 (SPSS Inc., Chicago, IL, USA). Tukey’s test after one-way ANOVA was used to determine significance differences in soil parameters and soil enzyme activities in each sample. Growth, biomass, DOC, SUVA, phenolic compounds and phenol oxidase activity were tested for significance by a t-test between ambient and elevated CO2 (P < 0.05).
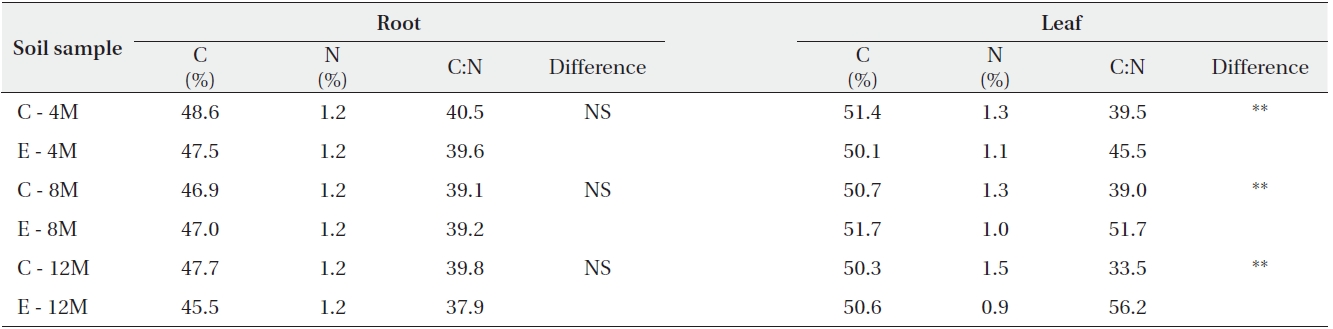
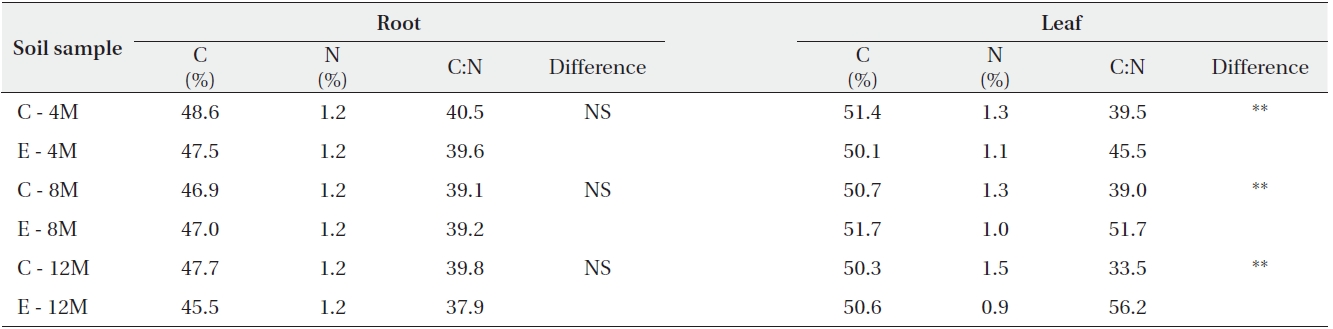
Carbon concentration, Nitrogen concentration, and the C:N ratio of Pinus densiflora grown under ambient or elevated atmospheric CO2
>
The effects of elevated CO2 on the biomass and C:N ratios of pine
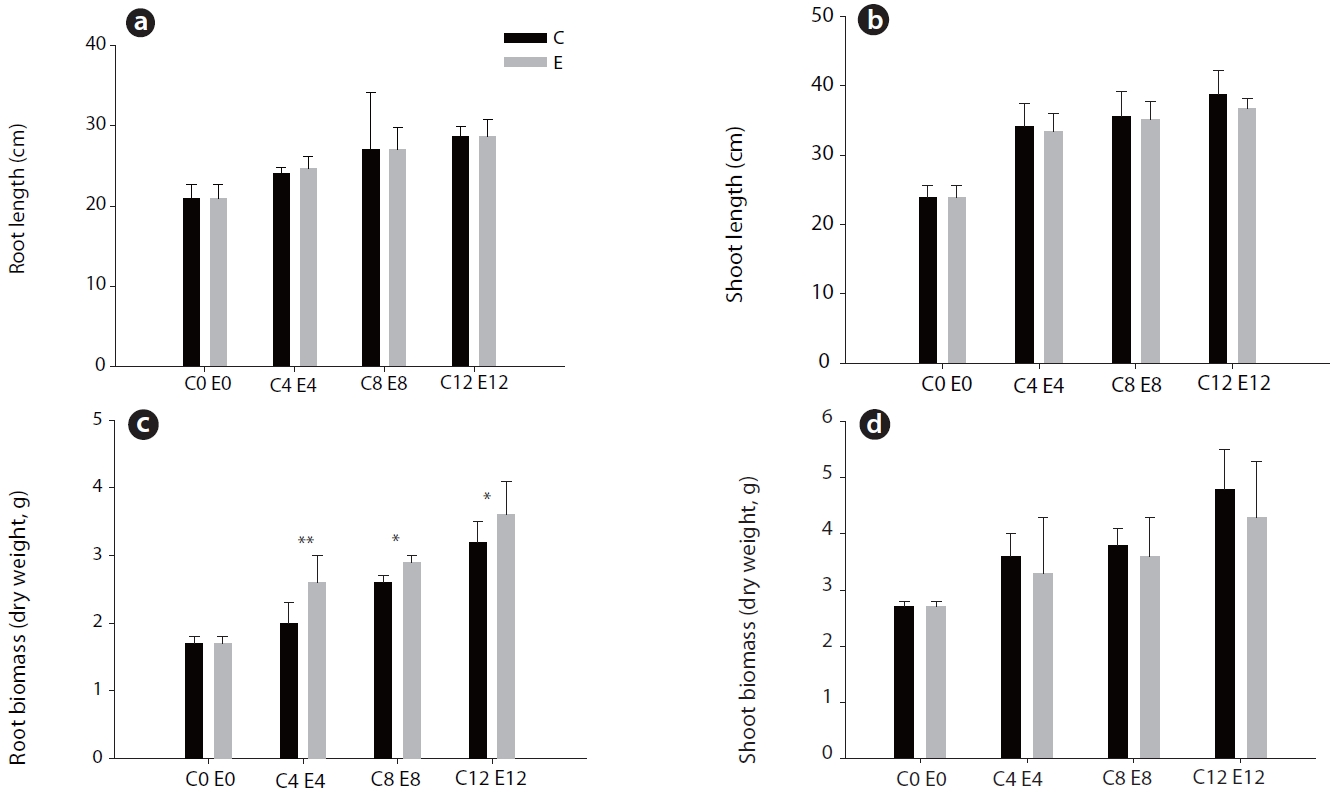
Although root and shoot elongation were not significantly affected by elevated CO2, the root dry weight of pine under elevated CO2 was increased (
>
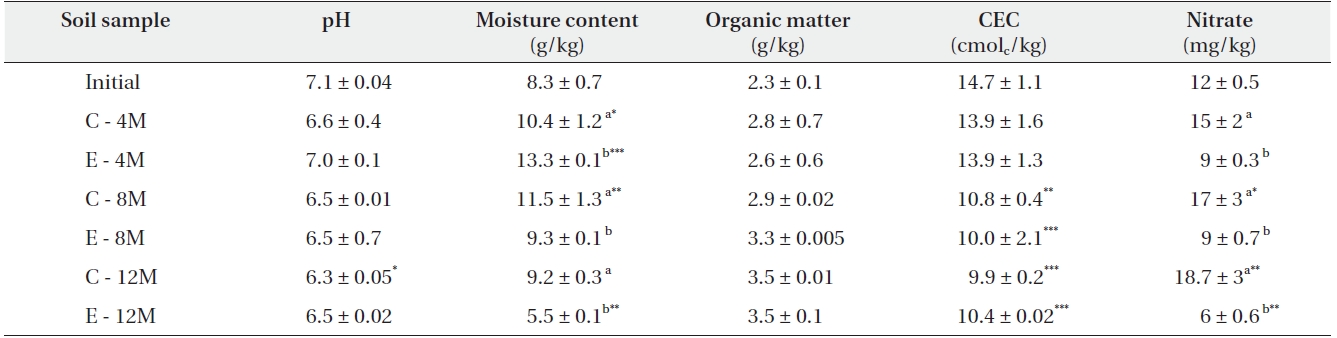
Comparison of soil physical and chemical characteristics
The physicochemical parameters of the soils are listed in Table 2. Under elevated CO2, both NO3- and water content were decreased in soil. The soil pH was found to be mildly acidic (6.3-7.1) while the organic matter content ranged from 2.3 g/kg to 3.5 g/kg.
>
Various levels of dissolved organic carbon, aromatic and phenolic compounds in soil
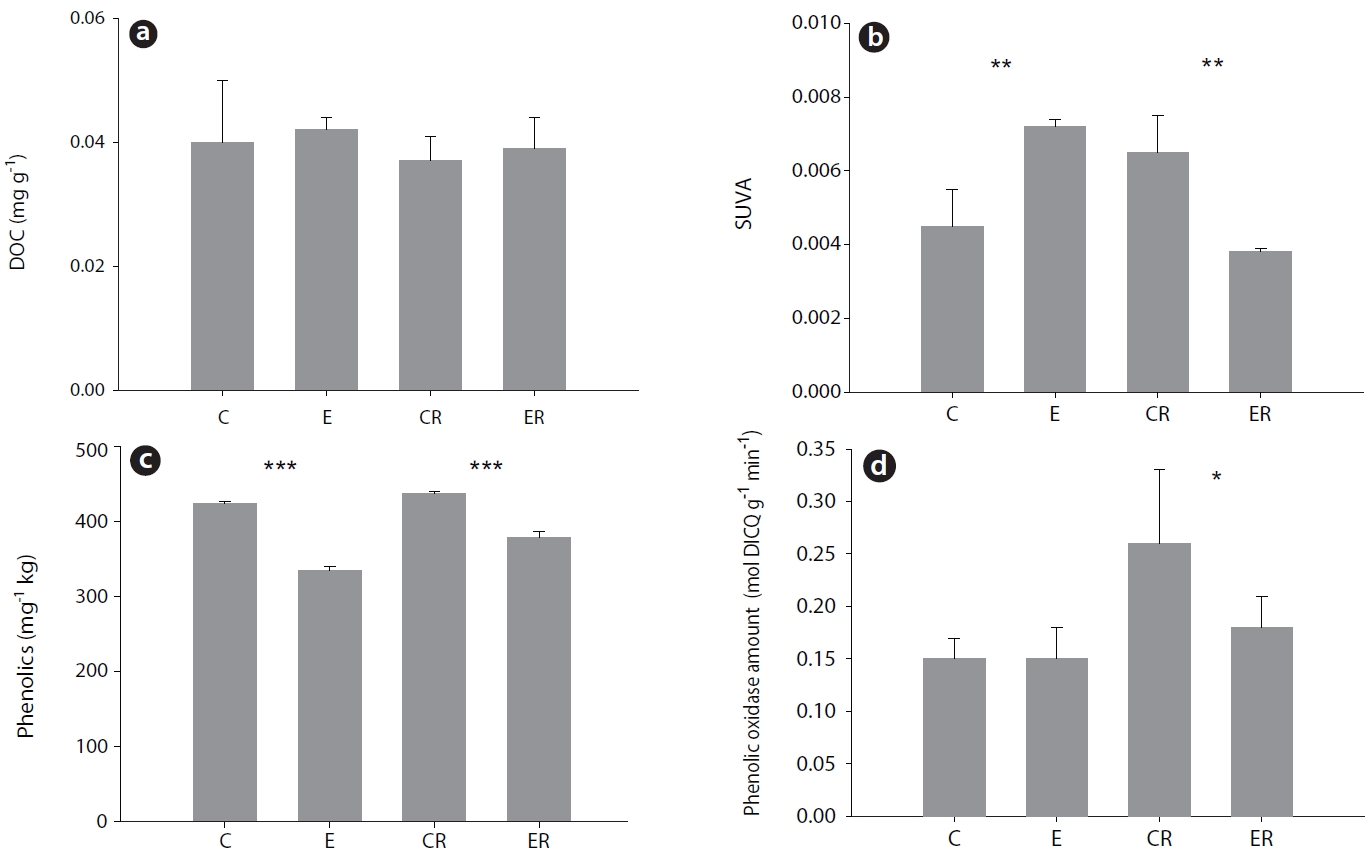
Elevated CO2 had no effect on DOC concentration in soil (Fig. 2a). However, the composition of DOC did appear to be influenced. Under elevated CO2, the proportion of aromatic material in DOC (estimated by SUVA254) was increased in bulk soil but decreased in rhizosphere soil (Fig. 2b). In contrast, phenolic compound content was decreased in both rhizosphere and bulk soil (Fig. 2c). Interestingly, phenol oxidase activity was decreased only in rhizosphere soil (Fig. 2d).
Elevated CO2 increased dehydrogenase activity (a measure of microbial intracellular activity) in bulk soil only (Table 3). β-glucosidase, N-acetylglucosaminidase, and phosphatase activities under elevated CO2 were increased in soils, especially rhizosphere soil (Table 3).
Elevated CO2 affected the C:N ratio of pine seedling as well as soil enzyme activity and N content. However, plant growth and soil DOC content were unaffected. Interestingly, elevated CO2 increased the C:N ratio of plants as well as microbial activities. It has been suggested that increased CO2 decreases the level of nitrate in soil. Elevated CO2 could likely decrease N into the soil, both of which are necessary for plant and microbial decomposition function. These decreases in soil nitrate levels are extremely important because net primary productivity is nitrogen dependent (Janus et al. 2005). Thus, as the amount of available N for plant uptake is decreased, the C:N ratios in pine needles are increased. Gifford et al. (2000) also similarly reported that the C:N ratio of pine seedling is increased 15% due to a 21% decrease in the N content of needles under elevated CO2.
[Table 2.] Soil physical and chemical parameters
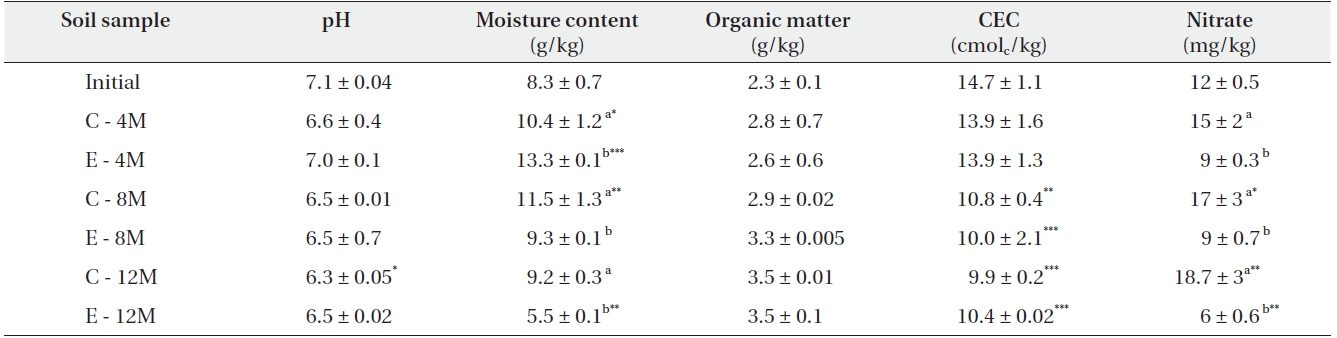
Soil physical and chemical parameters
[Table 3.] Enzyme activities in soil under ambient and elevated CO2
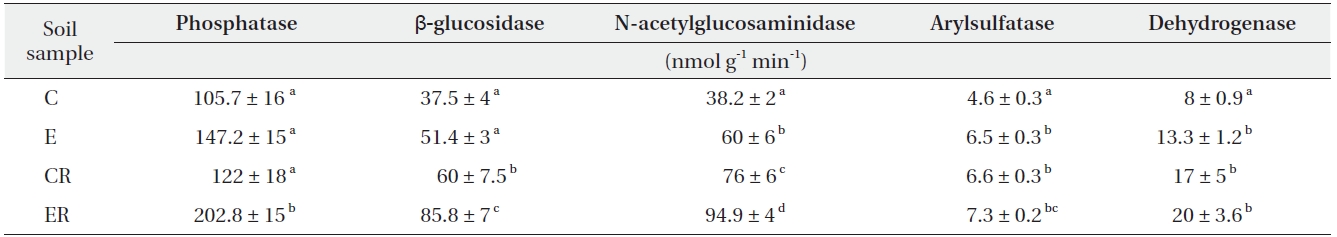
Enzyme activities in soil under ambient and elevated CO2
It was observed that the root dry weight of pine seedling was increased under elevated CO2, although root and shoot elongation were unaffected (Fig. 1). Many studies show that the overall biomass is more affected than either root or shoot growth under elevated CO2. Pushnik et al. (1999) reported that the root biomass of
The microbial activities of β-glucosidase, N-acetylglucosaminidase, and phosphatase were increased under elevated CO2 (Table 2), particularly in rhizosphere soil (
Elevated CO2 did not influence the concentration of dissolved organic matter in soil, but the concentrations of phenolic compounds in soil and aromatic compounds in rhizosphere soil were decreased (Fig. 2). Phenolic compounds are resistant to the nitrification of microbial decomposition activity. Therefore, elevated CO2 could promote their use as carbon sources by microbes (Rouhier and Read 1998). This notion is supported by our finding that phenol oxidase activity in soil was reduced under elevated CO2.
In this study, changes in the level of nitrogen in soil could explain the effects of elevated CO2 on microbial activities and pine seedling growth. However, as our work is limited to the growth chamber, further investigation is needed study.
The results of this study demonstrated that elevated CO2 had significant effects on the growth of pine seedling as well as soil microbial activity. These findings suggest that rising levels of atmospheric CO2 cause a reduction in pine seedling biomass as well as distinct changes in soil chemistry and microbiology.