The genera
Studies on the kelp flora from the Sea of Okhotsk, especially its northern areas, have proceeded slowly due to difficulties in transportations and harsh weather conditions. Moreover, the continental coast line of the northern areas of the Sea of Okhotsk is sparsely populated. Among early phycological research, remarkable contributions were made by Ruprecht (1850), Schapova (1948), Zinova (1953), Zinova (1954), Blinova (1968), Zinova et al. (1980), Petrov and Vozzhinskaja (1966, 1970), and Petrov and Suchovejeva (1976), who were among the pioneers of Russian phycology and provided the first floristic lists of seaweeds or described new and endemic species from the Sea of Okhotsk. More recent works have described new and endemic kelp genera and species from the Sea of Okhotsk (Klochkova and Krupnova 2004, Cho et al. 2006).
The Sea of Okhotsk was reported to be inhabited by several endemic species, including
>
Sample collection and specimen observation
One of the authors (M.N. Belij) has scuba-dived in various northern localities of the Sea of Okhotsk for many years, including the type locality of
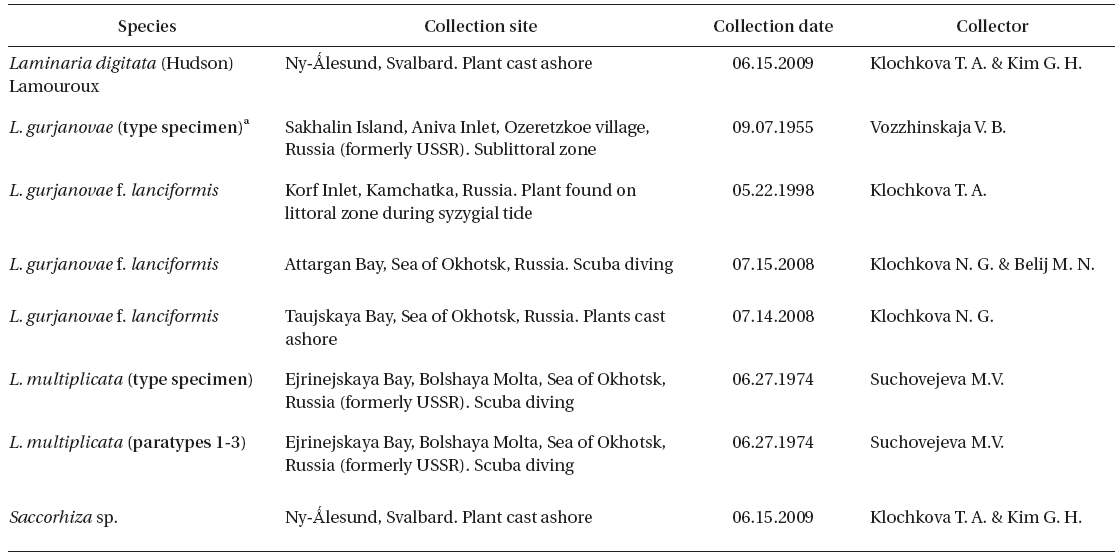
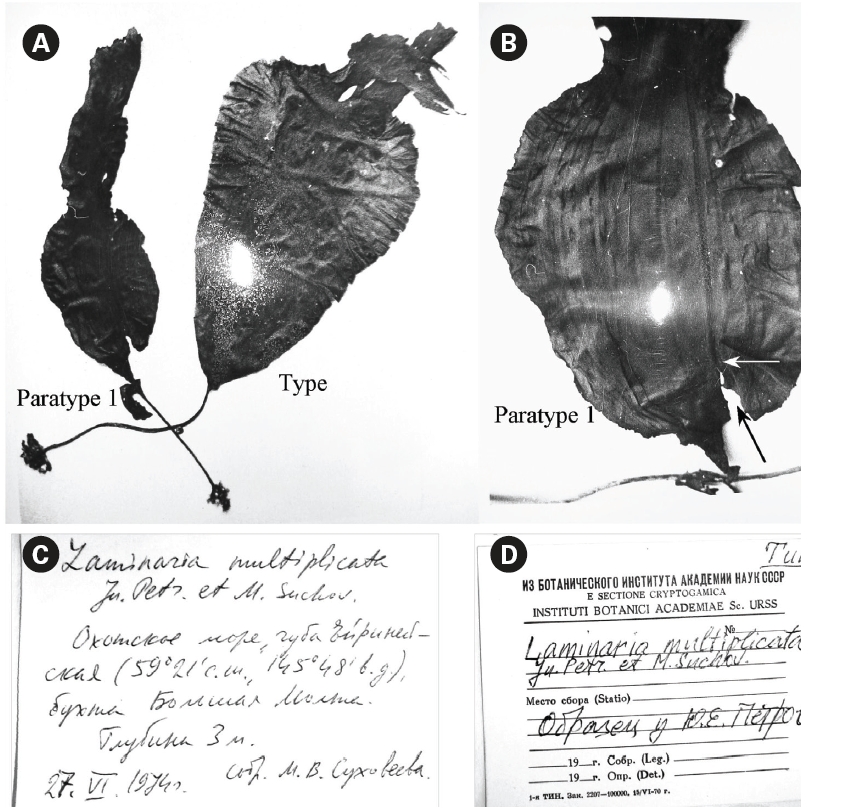
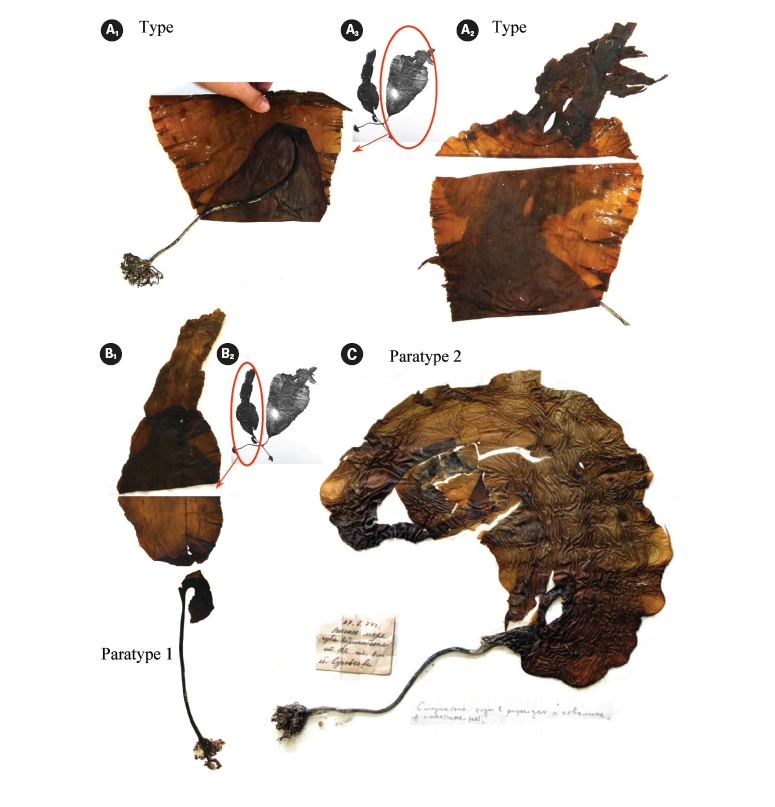
Of the eight plants collected in June 1974 (Petrov and Suchovejeva 1976), we studied dry specimens of three plants (type and two paratypes) that are currently held in the Komarov Botanical Institute of Russian Academy of Sciences (KBI RAS, Saint-Petersburg, Russia) and one piece of plant (paratype 3) kept by Dr. M. Suchovejeva in the Pacific Institute of Fishery and Oceanography (TINRO-center, Vladivostok, Russia). It is noteworthy to mention that the type and paratype 1 were not kept in the form they were displayed in Petrov and Suchovejeva’s paper (1976), but were re-hydrated again after initial photography, then folded and dried. The blade of paratype 1 was detached from its holdfast.
For microscopic observations, we used type and paratype of
Collections of dry specimens of Russian laminariaceaen algae kept in KBI RAS, TINRO-center, and Kamchatka State Technical University (KamchatGTU, Russia) were also studied. The list of all studied specimens is given in Table 1.
[Table 1.] List of specimens in this study
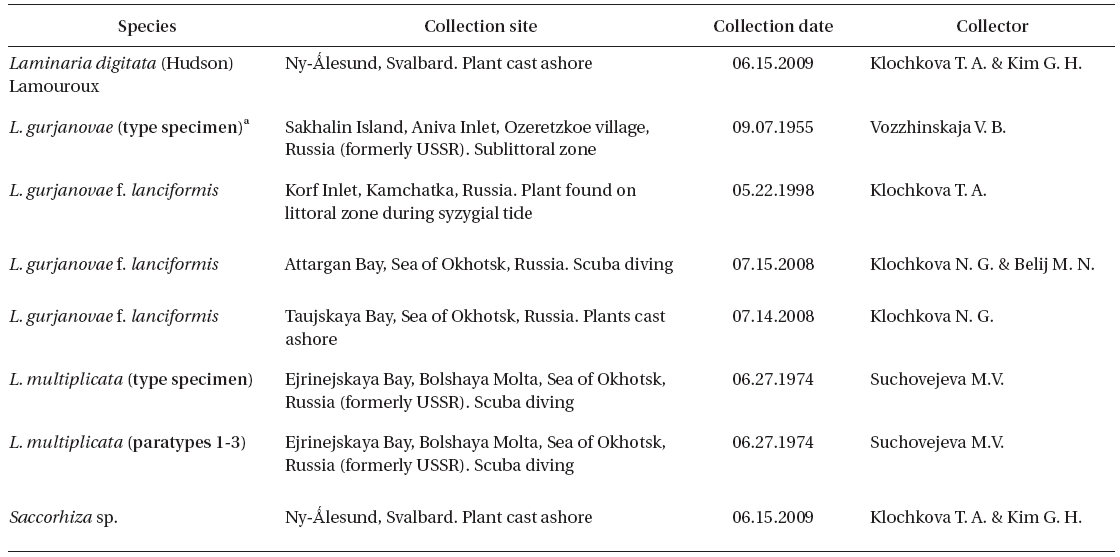
List of specimens in this study
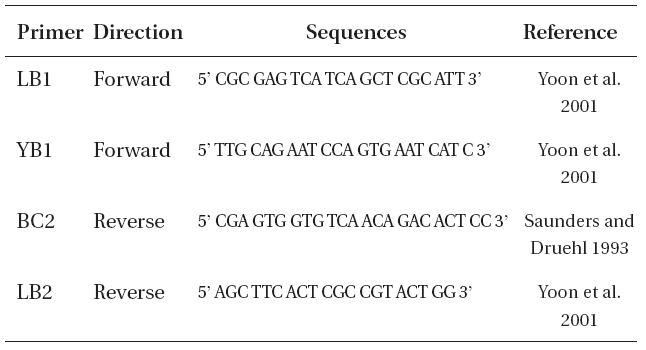
For DNA extraction, we used type of L. multiplicata from KBI RAS. DNA extraction was performed in January 2009, thus the material was approximately 35 years old. The DNA was extracted using 1) i-genomic DNA Extraction Mini Kit (iNtRON Biotechnology, Seongnam, Korea) following the manufacturer’s instructions and 2) hexadecyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1987). During the stages of incubation in 100% isopropanol (CTAB method), the samples were incubated at -20°C for 1-4 weeks. Polymerase chain reaction (PCR) was performed using primers displayed in Table 2, with an initial denaturation at 94ºC for 4 min, followed by 35 cycles of amplification (denaturation at 94ºC for 30 s, annealing at 45ºC for 30 s and extension at 72ºC for 1 min) with a final extension at 72ºC for 10 min.
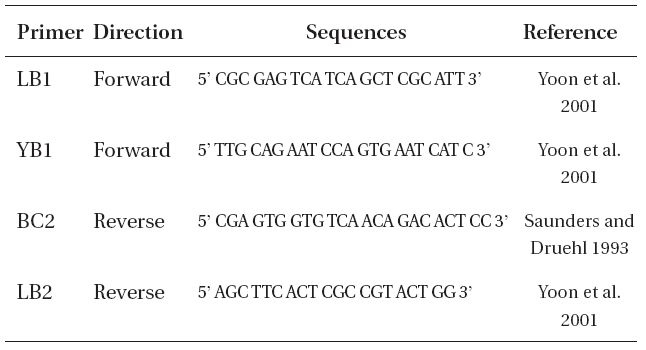
Oligonucleotide primers used to amplify internal transcribed spacer region in Laminaria multiplicata
>
Laminaria multiplicata Petrov et Suchovejeva
Basionym:
Synonym: None.
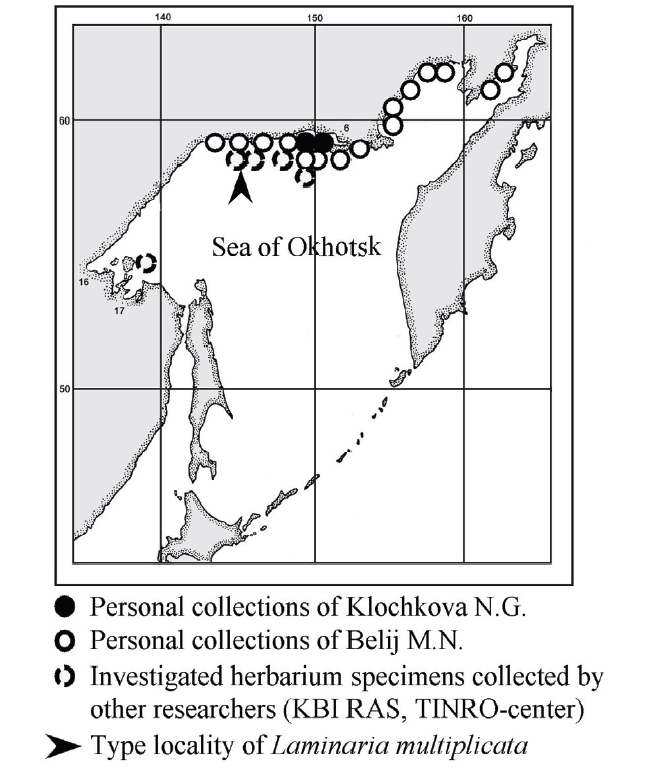
Type: Sea of Okhotsk, Ejrinejskaya Bay, Bolshaya Molta (59o22' northern latitude 145o48' eastern longitude, Figs 1-3), depth 3 m, 27.VI.1974, col. by Suchovejeva M.V., kept in Botanical Institute of Russian Academy of Sciences, Leningrad (currently KBI RAS, Saint-Petersburg).
Original description by Petrov and Suchovejeva (1976)in Russian language: In June 1974, the scuba divers of the scientific-research ship of the Pacific Scientific-Research Institute of Fishery and Oceanography (TINRO) have collected 8 plants, which were later named as L. multiplicata (Petrov and Suchovejeva 1976). The plants had elliptical or wide cuneiform blades of 56-102 cm long and 21-40 cm wide and up to 1.5 mm thick, with numerous narrow longitudinal folds (Rus.: складки). The folds were 0.5-33 mm in width and arose over the blades surface at 0.6 mm, making it thickened or sagged. The blades had 20-30 or more folds. In fresh plants, a wide middle stripe was distinguished. The blades’ edges had small waving. The medullar layer occupied 0.4-0.5 of the blade’s total width. Mucilage channels were present in the cortex layer and sometimes in the intermediate layer. Holdfast was thin, 17-28 cm long, with a ring of mucilage channels and lacunas; rhizoids had a ring of mucilage lacunas. Sporangia appeared as narrow and short lines on the folds, later forming spots on both sides of the blade coinciding in shape. This species grew at 3 m depth. It was similar to L. gurjanovae A. Zin. in 1) sporangia sori shape that coincided on both sides of the blade, 2) blade shape, and 3) length of holdfast. Perhaps, it originated from L. gurjanovae A. Zin., however differed from this and all other species of Laminaria by having numerous folds. However, it could not be attributed to Cymathere due to small thalli sizes and presence of numerous folds, and also because it was similar looking with one of the Laminaria species (herein: L. gurjanovae).
In this paper, we use the names L. gurjanovae A. Zinova (1964, 1969) and L. gurjanovae f. lanciformis Petrov (1972) instead of Saccharina gurjanovae (A. Zinova) Selivanova, Zhigadlova et Hansen and S. gurjanovae f. lanciformis (Petrov) Selivanova, Zhigadlova et Hansen throughout the text. The reason is as follows. Selivanova et al. (2007) stated that they re-named and re-authorized species and consecutively its form on the basis of molecular-phylogenetic data, but did not provide evidence of those data from 2007, such as sequences in GenBank (http://www.ncbi.nlm.nih.gov, searched on April 23, 2010). Also, their paper did not contain detailed methods used for molecular analysis or pictures of the plants. Omissions of these basic criteria make the nomenclatural combinations doubtful and the claims unsupported. Whoever provides trustable evidence such as sequences in GenBank of L. gurjanovae and L. gurjanovae f. lanciformis and proves their positioning in the Saccharina clade will be the author of new nomenclatural combination. Until then the case should remain open and the names existing until 2007 should be used, cf. Laminaria gurjanovae A. Zinova and L. gurjanovae f. lanciformis Petrov.
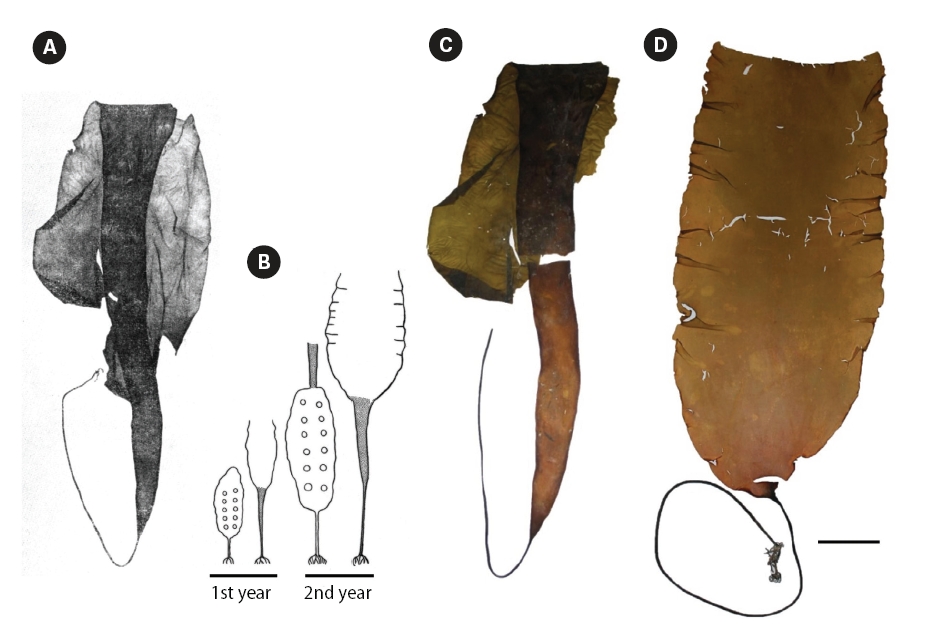
It is noteworthy to mention that description of L. gurjanovae f. lanciformis was given by Petrov (1972) in violation of the condition of valid publication of names according to International Code of Botanical Nomenclature (Art. 42, McNeill et al. 2006), since a diagnosis in Latin language was given and no illustration provided. Strictly speaking, the name should be regarded as nomen invalidium, although we still have used it in this paper. Figs 4D & 5A-C depict plants that have historically been
identified as L. gurjanovae f. lanciformis by Russian phycologists. We also present photograph of type specimen of L. gurjanovae collected from Sakhalin Island in 1955 (Fig. 4A & C). In our understanding, the plant chosen by Zinova (1969) as type specimen of L. gurjanovae was at the end of the second year of growth, based on the author’s scheme of thalli changes (Fig. 4B). When stating similarity of L. multiplicata to L. gurjanovae, Petrov and Suchovejeva (1976) did not mention any forms described by Petrov (1972) and did not specify which one resembled L. multiplicata. However, as seen from a schematic drawing by Zinova (1969), the morphology of L. gurjanovae (cf. L. gurjanovae f. gurjanovae, Petrov 1972) varies greatly according to age, and its thalli at the end of the first and beginning of the second years of growth do indeed look similar to L. multiplicata. Zinova (1964) also stated that young (1-year-old) plants of L. gurjanovae had wide cuneiform blades, whereas in older plants the lower part of blade was extended in length, becoming narrow cuneiform. Our field observations of L. gurjanovae and L. gurjanovae f. lanciformis also showed that morphologies could vary according to age and growth locality (Figs 4D & 5A-C).
>
Observations of available specimens of Laminaria multiplicata by the present authors
Petrov and Suchovejeva (1976) showed two plants in one photograph under the legend ‘General view of type specimen (a)’ without specifying the scale bar (Fig. 2A & B). One plant had the letter ‘a’ put close to it and, thus, was presumably a type specimen, whereas the second plant was presumably a paratype. To our knowledge, eight plants of this species (type and seven paratypes) have ever been collected since 1974. It was cited in floristic lists of the Sea of Okhotsk on the basis of Petrov and Suchovejeva’s paper (Emelyanova 2006). No additional descriptions, photographs, or micrographs have been published to date except for Petrov and Suchovejeva’s paper. Also, the latter study did not cite any reference to support their finding.
It is not known how many dry specimens were prepared from the eight plants collected by Suchovejeva in 1974. We were able to find three specimens of L. multipli-cata in KBI RAS (Fig. 3). Also, one piece of L. multiplicata was in the collection at the TINRO-center. Perhaps, the remaining four paratypes were held by Dr. Petrov. If so, they may be lost to study, since Dr. Petrov has not been an active researcher in the Russian Academy of Sciences from 1990-es. We found a type specimen (Fig. 3A1-3) and a broken specimen of paratype 1 (Fig. 3B1, 2) in KBI RAS. We analyzed them microscopically (Figs 6 & 7) and attempted to analyze the DNA.
Observation of morphology of available plants and analysis of the original photographs showed that some had development abnormalities. For instance, paratype 1 (Fig. 3B1-2) was torn longitudinally from its basal part (Fig. 2B) and the breaking point was right on the ‘fold’ line described by Petrov and Suchovejeva (1976). Paratype 2 (Fig. 3C) had a long holdfast and asymmetric blade bent to one side; moreover, it did not have the dark-colored projections/protuberances previously designated as folds (cf. Petrov and Suchovejeva 1976). We do not know what the remaining plants looked like, but, among those currently available, only the type looked more or less normal (e.g., with proportional and complete blade), and not appreciably different from a known species, L. gurjanovae, except for the so-called ‘folds’.
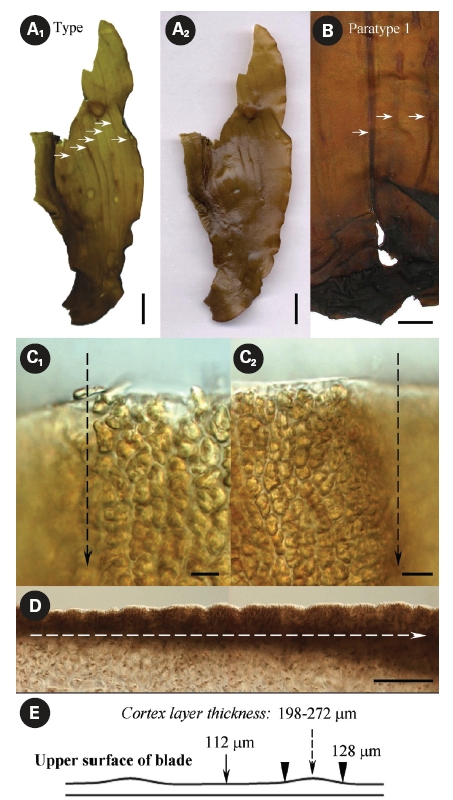
What are these folds? Petrov and Suchovejeva (1976) provided descriptions but did not show microphotographs of the structures they termed folds and used as key character of L. multiplicata. In the classical view, a fold is a line or an arrangement made by the doubling of one part over another. Our microscopic observations of L. multiplicata revealed that the darker lines on the blades’ surface, which were called ‘folds’ by Petrov and Suchovejeva, were in fact irregularities of the cortex layer’s thickness resulting in slight projections/protuberances on its surface (Fig. 6).
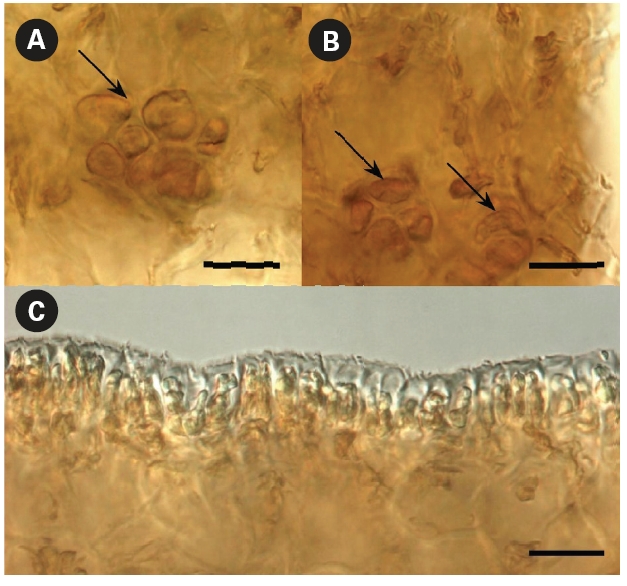
As appeared from the longitudinal preparations and cross sections of the blade, the thickness of the cortex layer made with brown-colored small cells differed from 112-128 μm to 198-272 μm in the most thickened and projected part, which created longitudinal protuberances on its surface (Fig. 6C-E). Because the brown-colored layer had different thicknesses and was more sloped upward in some places, they appeared darker. Moreover, physodes with concentrated brown pigment were found in places where the blade’s cortex layer was thicker (Fig. 7A & B). Physodes are rather common in the kelp species (Klochkova N.G., personal observations), contained as colorless vesicles in young cells and yellow and brown vesicles in old cells, and they contain tannins in the form of chloroglycin and other polyphenols (Petrov 1977).
The protuberances developed longitudinally (Fig. 6A & B) when the unevenness of the cortex thickness started in the blade’s basal part during the early stages of thalli growth and proceeded in the same areas when the blades
grew in height. They appeared only on one side of the blade and the reverse side did not have such distinct drops of cortex thickness, although its surface was slightly wavy (Fig. 7C). Of course, one might question that the plants were collected approximately 36 years ago and were hardly pressed during specimen preparation. However, if those structures on the surface were indeed numerous narrow longitudinal folds of 0.5-33 mm at width, they would have been dried folded, whereas they were all dried unfolded and slightly projected. Moreover, they were very distinguishable in the dry and re-hydrated thalli, even after almost four decades of preservation. Thus, our microscopic observations show that the structures termed folds, which were the key character of this species, are in fact protuberances resulting in irregularities in the blades’ texture. No other key characters were listed by Petrov and Suchovejeva (1976) to distinguish this species from other Laminaria spp. Also, they mentioned that L. multiplicata might have originated from L. gurjanovae because of similarities in sporangia sori shape that coincided on both sides of the blade, the blade shape, and holdfast length. L. gurjanovae is an independent species described by Zinova (1964, 1969) who mentioned it from the Sea of Okhotsk, Sea of Japan, and Sakhalin.
Fig. 5A shows L. gurjanovae f. lanciformis collected from Korf Inlet, Kamchatka. This plant had similar holdfast, rhizoids, and blade shape to L. multiplicata. It also had a single longitudinal protuberance starting from the blade’s base and proceeding along the entire length; it was present on one side of the blade, appearing in the same manner as in L. multiplicata except that in the latter species they were numerous. We previously observed similar irregularities in the blade texture in laminariaceaen algae, especially in plants developing under the influence of anthropogenic pollution (Klochkova N.G., personal observations). We also noted similar stripes in L. gurjanovae f. lanciformis from the Attargan Bay and Taujskaya Bay in the Sea of Okhotsk (Fig. 5B-D). So, we believe those were developmental abnormalities. As can occur with any plant, kelps may develop various abnormalities (Fig. 5) (Klochkova and Berezovskaya 2001). However, it should not have been mistaken for a key character to describe a new species, L. multiplicata.
The on-site collection activities of two of this paper’s authors have been described earlier. They collected numerous Laminaria, Saccharina, and other kelp species from the Sea of Okhotsk, including rare or endemic species, but none resembling Petrov and Suchovejeva’s description of L. multiplicata, even in the type locality. There were abundant kelp beds on the bottom of the Ejrinejskaya, Penzhinskaya, Gizhinskaya, and Taujskaya bays. Moreover, they found abundant plants of the endemics of the Sea of Okhotsk such as L. appressizhiza, L. inclinatorhiza, T. basicrassa, and etc., but not a single plant resembling description of L. multiplicata.
Kelp species such as Laminaria and Saccharina do not tend to grow as a single isolated plant on a large space of sea bottom, but are rather found in clusters or form beds (e.g., Klochkova and Berezovskaya 1997, Klochkova et al. 2009). They are also perennial plants. Medium-sized kelp such as L. multiplicata (cf. 56-102 cm long and 21-40 cm wide) is impossible to miss when one targets it for several consecutive years like we did, and yet it has not been found by anyone since 1974.
We tried to analyze the DNA of type specimen of L. multiplicata using conventional extraction with Kit and the CTAB method. Conventional method has failed to yield any DNA, but the CTAB method was partially successful. The amplification of only one internal transcribed spacer region was possible but the result was unclear and did not allow for credible comparison. Thus, the DNA of currently existing specimens of L. multiplicata is already damaged and cannot be studied. There could be many reasons for the damage, including 1) material age (ca. 35 years-old at the time we analyzed it), 2) initial preparation of collected samples (rinsing in freshwater for mannitol removal, fast air drying, presence/absence of formalin treatment after collection), and 3) preservation conditions during the past 36 years. It is noteworthy that we have previously obtained clear DNA results from 20-year-old brown algae using the CTAB method. Thus, the method is applicable to old algal samples. Whatever the reason, the DNA of the plants described as L. multiplicata could not be studied. The data files of fragmentary sequences and remaining dry sample are available upon request from the first author; however incomplete sequences have not been registered in any database.
We strongly doubt the existence of