The genus Centroceras was described by Kutzing (1841) without designation of a type, and subsequently was lectotypified with C. clavulatum (C. Agardh) Montagne by Kylin (1956). Centroceras as it is currently understood is composed of 12 species: Centroceras clavulatum, C. distichum Okamura (1934), C. corallophiloides R. E. Norris (1993), C. gasparrinii (Meneghini) Kutzing (1849), C. hyalacanthum Kutzing (1841), C. internitens Gallagher & Humm (1983), C. japonicum Itono (1973), C. micracanthum Kutzing (1841), C. minutum Yamada (1944), C. rodmanii Won, T. O. Cho et Fredericq (2009), C. secundum Wynne (2003), and C. tetrachotomum Won, T. O. Cho et Fredericq (2009).
The type species of Centroceras, C. clavulatum, was widely known as a prime example of a cosmopolitan species by most phycologists (e.g., van den Hoek and Breeman 1990). It had been viewed as highly variable morphologically and a ubiquitous species (Hommersand 1963). However, recently, Won et al. (2009) distinguished this species into several discrete species and restricted it to specimens with pseudo- or trichotomous branching patterns, straight spines, fattened gland cells and tetrasporangia without involucral branchlets. It was also mentioned that C. clavulatum has a restricted distribution range in northern Chile, Peru, Southern California, South Australia, and New Zealand.
After the first record of "Ceramium clavulatum" was made from Busan by Okamura (1936), "Centroceras clavulatum" has been reported from all coasts of Korea including Guryongpo (Kang 1966), Gampo (Boo and Lee 1985), Wando (Kang 1966) and Jeju (e.g., Kang 1960, Lee 2008). It has been known as a very common species of Korean marine flora.
Centroceras gasparrinii was described by Meneghini (1844) as "Ceramium gasparrinii" and synonymized with Centroceras clavulatum by Agardh (1851). Recently, Centroceras gasparrinii has been resurrected from C. clavulatum by Won et al. (2009) with the following diagnostic characters: a gland cell and a cortical cell, a spine and a cortical cell, or two cortical cells produced from the first cortical initial; an ovoid cortical cell on the second cortical initial; straight spines; ovoid gland cells; tetrasporangia with involucral branchlets. Won et al. (2009) reported this species from east coast of Korea.
To clarify the entry of Centroceras clavulatum in Korean flora, several samples known as "C. clavulatum" were collected from Jeju, Jindo, Guryongpo, and Gampo in Korea and studied. This study confirms that C. clavulatum is excluded from Korean flora and C. gasparrinii is included instead.
According to previous characters of "Centroceras clavulatum", five Centroceras samples were collected from Jeju (TC6124, Seongsan, Jul. 23. 2008, T.O. Cho, S.J. Jeong, and J.K. Lee, TC6169, Hado Beach, Jun. 20. 2007, B.Y. Won and T.O. Cho), Jindo (TC6721, Baekdongri, Imhoemyeon, Aug. 20. 2009, T.O. Cho, S.J. Jeong, and J.K. Lee), Gampo (TC034, Sep. 27. 1998, S.M. Boo and T.O. Cho), and Guryongpo (TC127, Jul. 7. 1999, T.O. Cho), Korea and were either liquid-preserved in 5% Formalin/seawater or dried in Silica gel. Microscopic observations were made from materials stained with 1% aqueous aniline blue acidified with 0.1% diluted HCL (Tsuda and Abbott 1985). Photomicrographs were taken with an Olympus DP71 digital camera (Olympus Inc., Tokyo, Japan) attached to an Olympus BX51 microscope (Olympus Inc.). Voucher specimens used in this study are deposited in the herbarium of Chosun University, Gwangju, Korea.
Genomic DNA was extracted from silica-gel samples using the G-spinTM Iip genomic DNA extraction kit (iNtRON Biotechnology, Inc., Seongnam, Korea). The rbcL gene was amplified using the primer combinations F7-R753 and F645-Rrbcst as listed in Lin et al. (2001) and sequenced with the primers F7, F645, F993, R376, R753, R1150, and RrbcStart (Freshwater and Rueness 1994, Lin et al. 2001, Gavio and Fredericq 2002). PCR and sequencing sequencing protocols are as described in Cho et al. (2003). Sequences were determined for both forward and reverse strands using the ABI Prism 3100 Genetic Analyzer (PE Applied Biosystems, Foster City, CA, USA).
All generated sequence data of rbcL were compiled and the sequences aligned in the Genetic Data Environment (GDE 2.2) program (Smith et al. 1994). Maximum likelihood analyses were performed using PAUP*4.0b10 for Macintosh and UNIX (Swofford 2003). For maximum likelihood, a likelihood ratio test using MODELTEST 3.06 (Posada and Crandall 1998) was performed to determine the best available model for the rbcL data. Maximum likelihood analyses (heuristic search with random stepwise addition and 10 repetitions) were performed using the GTR model. Bootstrap analysis was conducted by performing replicate maximum likelihood searches, with random stepwise addition of sequences, using the same search conditions as described above. Five hundred bootstrap replicates for rbcL data were successfully performed.
Centroceras gasparrinii (Meneghini) Kutzing 1849 참가시비단풀
Vegetative plants
Thalli grow on rocky areas in the intertidal zone. Thalli grow up to 7 cm high, form dense tufts (Fig. 1A), and consist of erect and prostrate axes. Branching is pseudodichotomous (Fig. 1B) to trichotomous and, rarely, tetrachotomous. Branches are formed at intervals of 10-13 axial cells in the main and lateral axes. Adventitious branches develop from periaxial cells. Erect axes have forcipulate apices (Fig. 1B). The mature cortex is complete. Twelve periaxial cells are cut off obliquely from each axial cell and remain at the nodes (Fig. 1E & F). All periaxial cells (except the abaxial one) produce 3 corticating initials (Fig. 1G & I). Each corticating initial divides into cortical filaments contributing to the cortex. The first cortical initial acropetally cuts off a spine and a cortical cell (Fig. 1G), a gland cell and a cortical cell (Fig. 1H), or two cortical cells. The second cortical initial cuts off one cell acropetally and a filament basipetally by transverse division (Fig. 1G-I). The third cortical initial cuts off a filament basipetally (Fig. 1G-I). Acropetal cortical filaments are two cells long, including the cortical cell initial. Whorled spines occur at each node (Fig. 1B & C). Each spine is straight and three cells long (Fig. 1G) including the cortical initial. A gland cell is obliquely cut off from the first cortical initial and is ovoid in shape (Fig. 1H & I). Rhizoids are numerous, uniseriate, and multicellular and are produced from periaxial cells on the prostrate (Fig. 1D).
[Fig. 1.] Specimen (TC6721) of Centroceras gasparrinii from Baekdongri, Imhoemyeon, Jindo, Jeollanamdo, Korea. (A) Thallus. (B) Apical region. (C) Whorled arrangement in middle part of thallus. (D) Lower part of thallus showing rhizoid produced from periaxial cells. (E-F) Cross section views through cortical nodes of upper (E) and middle (F) parts of thallus. (G) Cortical unit showing the first cortical initial bearing a spine and a cortical cell. (H) Cortical unit showing the first cortical initial with both an ovoid gland cell (arrow) and a cortical cell. (I) Mature cortication showing cortical filaments with gland cell (arrows) developed from periaxial cells. (J) Tetrasporic thallus. (K-L) Cortical nodes of upper (K) and middle (L) parts showing tetrasporangia with involucral branchlets (arrows) in the abaxial side of axis. (M) Cortical nodes of lower part showing tetrasporangia with involucral branchlets (arrow) in whorl around the axis. Scale bars represent: A, 1 cm; B, C, K, 100 μm; D, 250 μm; E, F, L, M, 50 μm; G, H, I, 25 μm; J, 1 mm. Ax, Axial cell; C1?3, Sequence of cortical initials; P, Periaxial cell; T, Tetrasporangium; R, Rhizoid.
Tetrasporangia are distributed along upper cortical nodes of the erect axes (Fig. 1J). They are produced initially on the abaxial side (Fig. 1K & L), then on the adaxial side resulting in a whorl around the axis (Fig. 1M). A tetrasporangium is tetrahedral, spherical to ellipsoidal, and 42-55 μm in size. It is surrounded by involucral branchlets that are produced from the first cortical initials or are transformed from spines (Fig. 1K-M). Male and female thalli were not found in our collections.
Phylogenetic analysis
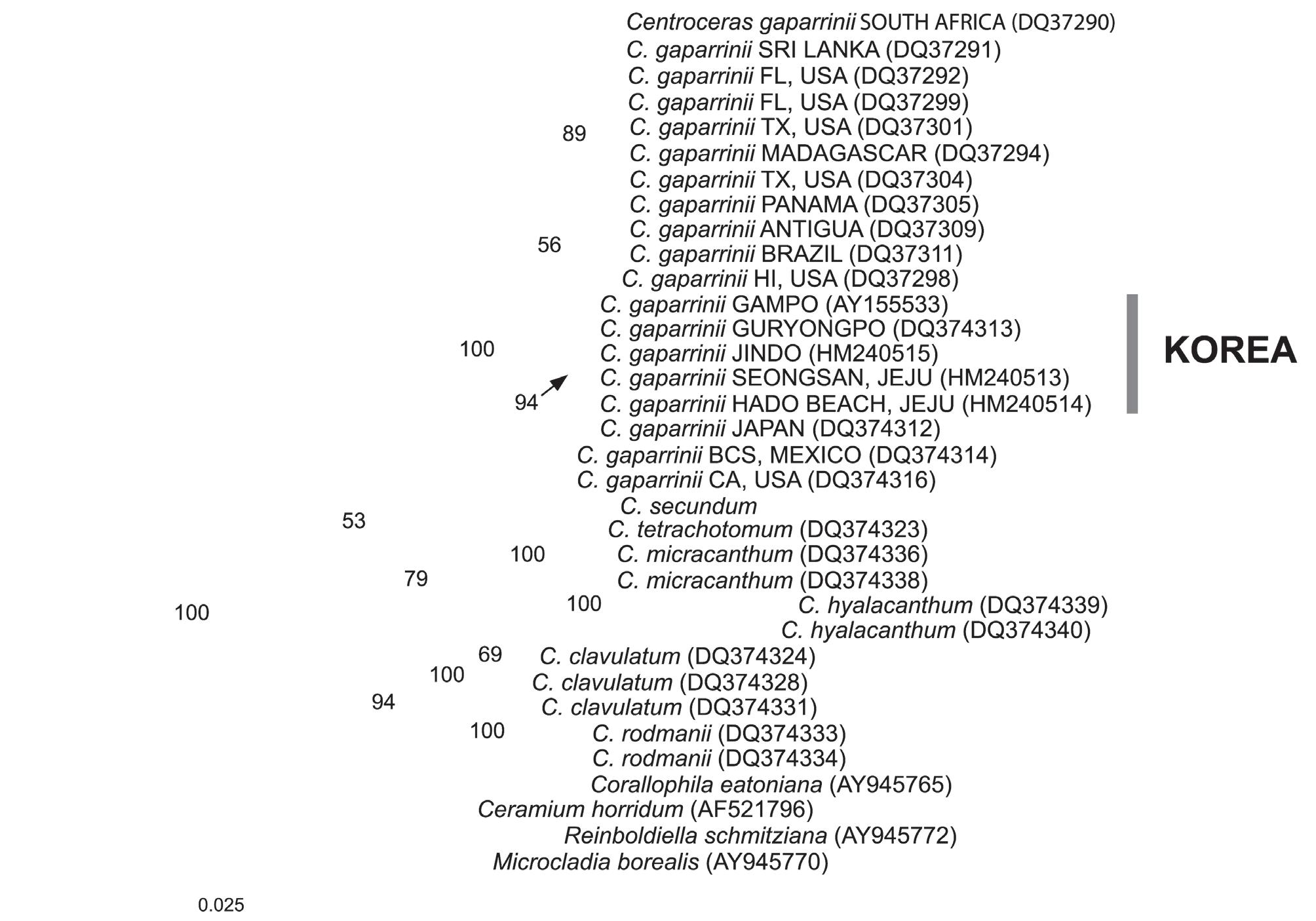
A 1412-bp portion of the 1467-bp rbcL gene (95.43% nucleotides sequenced) was sequenced that included 264 parsimony informative sites, excluding the outgroup. Pairwise distance showed that there is 0-0.07% sequence divergence among populations of C. gasparrinii from Korea. C. gasparrinii from the east coast of Korea differed by 1 bp from specimens from the south coast and Jeju Island. Intraspecific pairwise comparison of the rbcL sequences reveals 0-1.1% sequence divergence in Centroceras gasparrinii. Among Centroceras gasparrinii from Korea and Texas in USA, 16-17 bp (1.1%) were different in rbcL. The phylogenetic trees were obtained from the alignment of the rbcL sequences newly generated and downloaded from GenBank. Ceramium horridum, Corallophila eatoniana, Microcladia borealis, and Reinboliella smitziana in the Ceramieae were selected as the outgroup. Topologies of maximum likelihood (ML) (Fig. 2) and maximum parsimony (data not shown) of the chloroplast encoded rbcL are congruent except for the phylogenetic position of C. hyalacanthum, C. micracanthum, C. rodmanii and C. clavulatum.
Specimens from Korea correspond in their detailed morphological characters to the description of C. gasparrinii in Won et al. (2009). All morphological characters such as ovoid gland cells, straight spines, ovoid acropetal cortical cells, and tetrasporangia with involucral branchlets conform to those of C. gasparrinii. In addition, molecular evidence based on rbcL sequences also shows that specimens known as "C. clavulatum" with dichotomous branching patterns from Korea are C. gasparrinii.
To identify each species in Centroceras, Won et al. (2009) indicated that the vegetative features of Centroceras such as the shape of the acropetal cortical cells, the number of acropetal cortical cells and the shape of gland cells and spines are more important than previously used features. The importance of gland cell features has been viewed as one of the diagnostic characters in Centroceras, and its potential taxonomic merit was reported by Gallagher and Humm (1983) and Won et al. (2009). Two different shapes of gland cells were recognized: ovoid in C. gasparrinii, C. hyalacanthum, C. micracanthum versus flattened in C. clavulatum, C. rodmanii, C. tetrachotomum (Won et al. 2009). Won et al. (2009) also described two different types of reproductive structure: tetrasporangia with involucral branchlets in C. gasparrinii and without involucral branchlets in C. clavulatum, C. hyalacanthum, C. rodmanii and C. tetrachotomum. Involucral branchlets rarely occur in tetrasporic plants, but are commonly exhibited in female plants in Ceramiaceae. Although tetrasporangial involucral branchlets have not been emphasized as diagnostic characters for Centroceras species, they may be key characters for C. gasparrinii. Although C. gasparrinii is similar to C. clavulatum in that they both have the dichotomous branching pattern, the arrangement of cortical filaments, the straight spines and the ovoid cortical cell, the former is distinguished from the latter by having ovoid gland cells and tetrasporangia with involucral branchlets. Centroceras species with dichotomous habit from Korea has been recognized as C. clavulatum (e.g., Okamura 1936, Kang 1966, Boo and Lee 1985, Lee 2008). However, the results in this study show that all Centroceras collections with dichotomous branching patterns from Guryongpo, Gampo, Jindo, and Jeju, Korea are not C. clavulatum, but C. gasparrinii having ovoid gland cells and tetrasporangia with involucral branchlets.
In addition, newly generated sequences of rbcL gene reveal that all taxa of Centroceras collected from Korea are C. gasparrinii. Five sequences of Centroceras species from Korea are clustered in a group and form a monophyletic group with sequences of C. gasparrinii downloaded from GenBank. There is 0-0.07% sequence divergence among specimens of C. gasparrinii in Korea.
Centroceras gasparrinii is widely distributed throughout the Pacific Ocean (including Korea), Atlantic Ocean, Indian Ocean, the Gulf of Mexico, and Caribbean Sea, while C. clavulatum is restricted to northern Chile, Peru, Southern California, S. Australia, and New Zealand (Won et al. 2009). In Korea, Centroceras gasparrinii was reported from the east coast of Korea (Won et al. 2009) and its distribution range extends to the south coast and Jeju Island in this study.