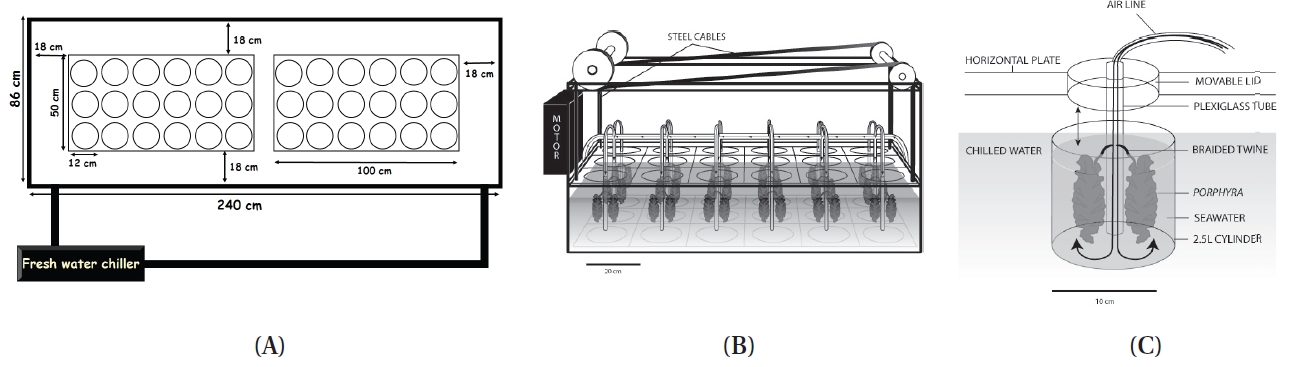
A tide-simulating apparatus was developed for culturing marine macroalgae. The objective of this study was to introduce a novel tide-simulating apparatus that can simulate a diurnal or semi-diurnal tidal cycle in the laboratory. In this apparatus, the seaweeds are move up and down and the water level remains the same during the simulated tidal cycle. The apparatus consists of 18 cylindrical culture tanks (3 blocks × 6 culture tanks) with 12 cm diameter and 24.5 cm long containing up to 2.5 L of seawater. There is a horizontal plate which covered all 18 culture tanks, and it is raised and lowered by a programmable motor that can regulate exposure time. In one application, seaweeds are attached to braided twine hung on Plexiglas air-tubing. The air-tubing is attached to a lid that is set on a horizontal plate. This apparatus is made of colorless Plexiglas to maximize light transmittance. This apparatus is easily disassembled and transportable to any indoor laboratory, wet laboratory, greenhouse, etc. This apparatus also offers considerable flexibility in terms of design. The size of culture tank can be redesigned by either increasing the height of cylinder or/and using a different diameter of cylindrical Plexiglas, therefore, larger/taller thalli can be cultivated. Growth rates of three eulittoral Porphyra species from different tidal elevations have been compared using this device
Eulittoral zonation is the distinctive pattern of an organism’s vertical distribution in the marine environment. Marine ecologists and physiologists have studied eulittoral zonation for over a century (Baker 1909, 1910, Lewis 1964, Dring 1992). The upper tidal limits of species is generally believed to be determined by their tolerances to physical environmental stresses associated with emersion, while the lower limits are thought to be determined mostly by biological processes such as competition and predation (Baker 1909, 1910, Lewis 1964, Dring 1992). However, there have been and are still many arguments to explain emersion effects on eulittoral seaweeds (Zaneveld 1969, Johnson et al. 1974, Quadir et al. 1979, Dring and Brown 1982, Chapman 1986, Johnston and Raven 1986, Thomas et al. 1987, Hurd and Dring 1990, Lipkin et al. 1993, Gao et al. 1999, Kim et al. 2008, 2009). For example, Zaneveld (1969) hypothesized that drought resistance determines the vertical distribution of seaweeds. Desiccation appears to enhance short-term uptake of nitrate, ammonium and phosphate in some eulittoral species (Thomas et al. 1987, Hurd and Dring 1990). Other studies have reported that the photosynthetic rate of eulittoral seaweeds remains relatively constant or even increases at the beginning of emersion and then declines with increasing water loss (Johnson et al. 1974, Quadir et al. 1979, Dring and Brown 1982, Ji and Tanaka 2002). Finally, the rates of recovery
of physiological functions such as photosynthesis and nutrient uptake following desiccation appear to be most important in determining the vertical distribution of seaweeds (Dring and Brown 1982, Johnston and Raven 1986, Gao et al. 1993, Lipkin et al. 1993, Kim et al. 2008). Many laboratory studies on the emersion effects have used a manual exposing method (Quadir et al. 1979, Dring and Brown 1982, Johnston and Raven 1986, Hurd and Dring 1990, 1991, Gao et al. 1999, Kim et al. 2009), which requires intensive labor and time. Therefore, most of those studies were short term experiments that might not allow seaweeds to fully or properly acclimate to the emersion effect in the laboratory. There have been a few other attempts to develop a tide-simulating apparatus to culture eulittoral seaweeds and sessile animals under controlled laboratory condition (Bracher 1919, Townsend and Lawson 1972, Underwood 1972, Allender 1977, Edwards 1977). Basically, two types of apparatus have been designed. In one approach, the “shore” is stationary and the water moves up and down (Underwood 1972) and the other type is where the “shore” itself moves up and down as a tidal cycle (Townsend and Lawson 1972, Edwards 1977). The former model would be most useful for marine benthic animals, whereas the latter would be most useful for benthic marine algae due to fact that animals could move, but seaweeds can’t. However, these early systems were small in scale. Most were primarily for algal sporelings and they failed to include independent tanks to satisfy the requirements for statistical replication. The Edwards (1977) apparatus had one nylon fishing line in the middle of a Plexiglas frame that was raised and lowered by a geared pedal system. The frame had several shelves that had slots to hold pseudo-replicate slides in one chamber. Another apparatus had two nylon threads and used four different diameter gear wheels connected to an electric motor to simulate different exposure times (Townsend and Lawson 1972). In the latter apparatus, balance had been a problem. To address problems with previous tide simulating systems, we designed a new system to enable the cultivation of mature macroalgae at different exposure levels. The objective of this study was to present a machine designed to produce a diurnal or semi-diurnal tidal cycles in the laboratory and to address
problems of scale inherent in previous tide simulating apparatus systems. We will report on the growth rates of 3 Porphyra species from New England that have been subjected to desiccation stress.
>
Description of the tidal apparatus
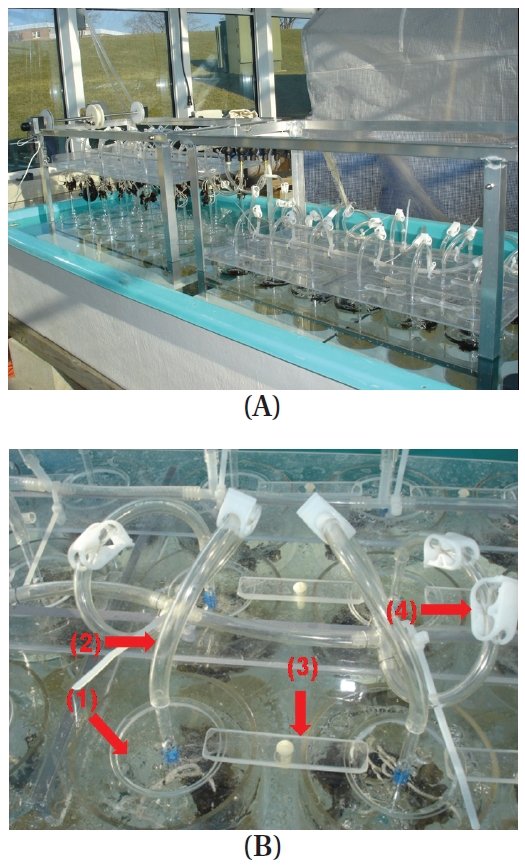
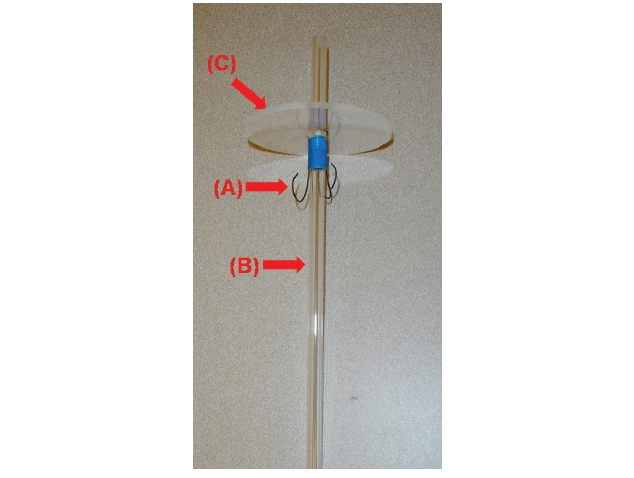
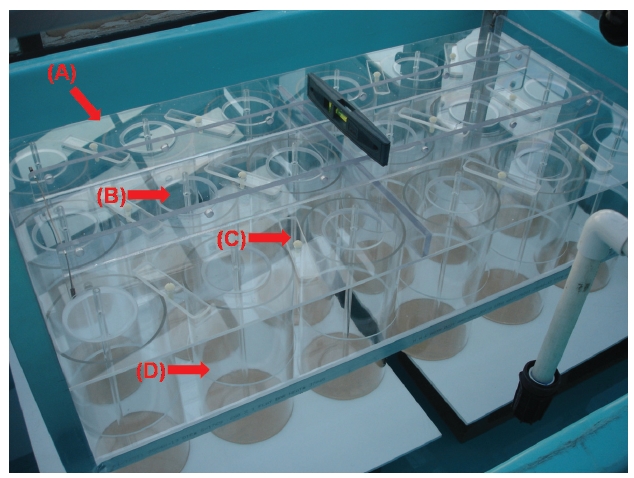
Unlike the tides where the water level periodically rises and falls, in this apparatus, the seaweeds are moved up and down while the water level remains the same during a simulated tidal cycle. The tide simulating apparatus consists of 18 independent cylindrical culture tanks (12 cm diameter, 24.5 cm long; 3 blocks × 6 culture tanks) each containing up to 2.5 L of seawater (Fig. 1). Seaweeds are attached to braided twine after a hole (0.5 cm in diameter) was punched in the middle of the blade (Appendix Fig. S1). The twine is hung on wire hooks that are attached to Plexiglas air-tubing (0.4 cm in diameter and 28 cm in length) (Appendix Figs S1 & S2). A horizontal plate covers all 18 culture tanks (Fig. 2, Appendix Fig. S3). There is one lid (7.5 cm in diameter and 0.25 cm in thickness) for each culture tank that is set on a horizontal plate (Fig. 2B, Appendix Figs S2 & S3). The lids are held by bars so that the lids remain tight over each chamber (Fig. 2B, Appendix Fig. S3). The horizontal plate is raised and lowered by a motor (model #: 2L004, 1.5 rpm, 12 VDC gear motor; Grainger Motor, Lake forest, IL, USA; www.grainger.com) (Appendix Fig. S4). The apparatus sits within a large fiberglass tank (240 cm in width × 86 cm in length × 40 cm in depth) containing up to 1,000 L of chilled freshwater to control the temperature of the culture tanks (Fig. 2A, Appendix Fig. S5). The culture tanks are made of cylindrical colorless Plexiglas (Appendix Fig. S3).
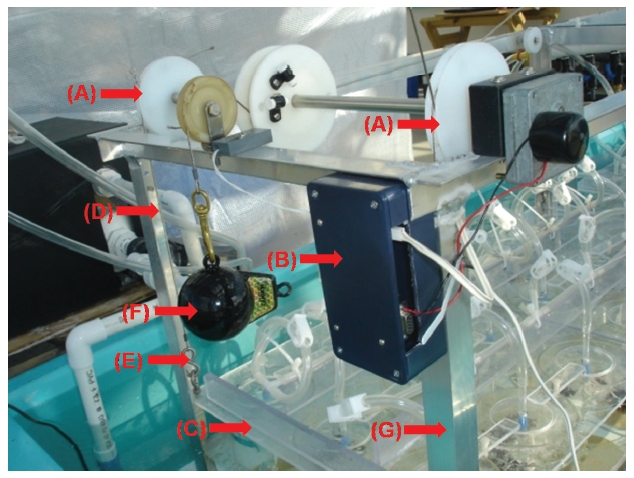
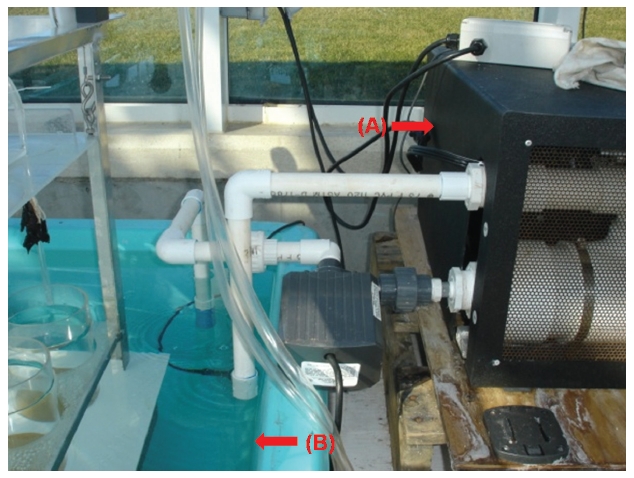
Exposure time of seaweeds is regulated by the geared programmable motor (Appendix Fig. S4). When the horizontal plate is raised and lowered, it remains level and does not bend. To insure the plate does not bend, a stainless steel cable (1.5 mm in diameter) is connected to each corner of the plate (Appendix Figs S4 & S6). The Plexiglas air tubing is suspended from a lid that sits on the horizontal Plexiglas plate (Fig. 2B, Appendix Figs S1-S3). The Plexiglas air tubing provides aeration for mixing and gas exchange of the culture medium (Figs 1C & 2B). The air flow is controlled using valves and clamps (1.3 cm O.D., Fig. 2B). A chiller (Delta Star®; Aqualogic Inc., San Diego, CA, USA; 1/2 hp) is connected to the large fiberglass tank to circulate the chilled water (Appendix Fig. S5). The Grainger Motor plugs into an UPS (model #: BE550R, Uninterrupted Power Supply; APC, West Kingston, RI, USA; 550 VA, 330 W) to avoid any unexpected power disruptions. The frame of the apparatus is made of aluminum (2.54 cm × 2.54 cm × 0.3 cm; Alloy 6061) that allows the motor, strings and aeration valves to be attached (Appendix Fig. S4). An identical apparatus without the motorized elevator is also in the large fiberglass chilling tank as a control (Fig. 2A). The whole system is installed in a greenhouse.
Porphyra leucosticta Thuret was collected from the lower eulittoral zone at Groton, Connecticut, USA (41º18′54.6˝N, 72º03′46.6˝) on March 29, 2007. Porphyra umbilicalis Kutzing was collected from the mid to upper eulittoral zone at Rye, New Hampshire, USA (43º00′43.3˝N, 70º44′04.6˝) on April 18, 2007. Porphyra yezoensis Ueda was collected from the sublittoral zone at Winnapaug Pond tidal cut, Weekapaug, Rhode Island, USA (41º19′43.8˝N, 71º45′46.7˝) on May 20, 2007. Experiments were carried out for 6 to 7 days in a greenhouse at the Rankin Laboratory of the University of Connecticut at Avery Point, Groton, CT. Samples were exposed twice per day by using a tide simulating apparatus.
The thalli were cultured in filtered (0.45 μm) seawater augmented with von Stosch’s enrichment (Ott 1965) without nitrogen and phosphorus. Nitrogen and phosphorus levels were 30 μM and 3 μM, respectively. Temperature of 10°C is reflective of what all species could experience in the field. The light intensity was measured by a Li-Cor 1000 sensor (Li-Cor Inc., Lincoln, NE, USA).
Biomass (fresh weight, FW) in each compartment was weighed 5-7 hours after re-submergence on the last day of experiments for all the species. Initial stocking density for each treatment was approximately 0.5 g L-1. Specific growth rate (SGR, expressed as % increase d-1) was calculated as follows:
where S1 and S2 are the FW at days T1 and T2, respectively. One-way split-plot ANOVA (α = 0.05) was used to analyze data. When ANOVA indicated treatment effect of desiccation, Tukey’s HSD analysis (α = 0.05) was used as a post hoc test to determine pairwise comparison probabilities between treatment level means. All statistic analyses were done using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Since the WL may vary depending on weather conditions, cloudless or partly cloudy days were chosen for experimentation. Fans distributed the air in the greenhouse and reduced the variance in the desiccation rate in all experimental compartments. WL of samples was controlled to 0, 40 ± 10% (2 h exposure), and 90 ± 5% (4 h exposure), WL was estimated as follows:
where FW is the FW obtained after blotting the thalli dry with paper towels. TW is the desiccated weight after known time interval. DW is the dry weight measured by drying a sample of the biomass at 60oC to constant weight.
>
Light, humidity and temperature
Light intensity, humidity and air temperature were measured every 30 minutes on the last day of each experiment. During mid-day (10:00AM-2:00PM), 30-55% of sunlight was blocked by the tempered glass of the greenhouse. The maximum light intensity measured during the experimental period was 1,200 ± 95 μmol photons m-2s-1 in the greenhouse. The air temperature and humidity during exposure were similar to the ones in the outside of the greenhouse due to the fact that all
the windows and doors in the greenhouse were opened during desiccation periods. The values were 15-27°C and 30-80%, respectively.
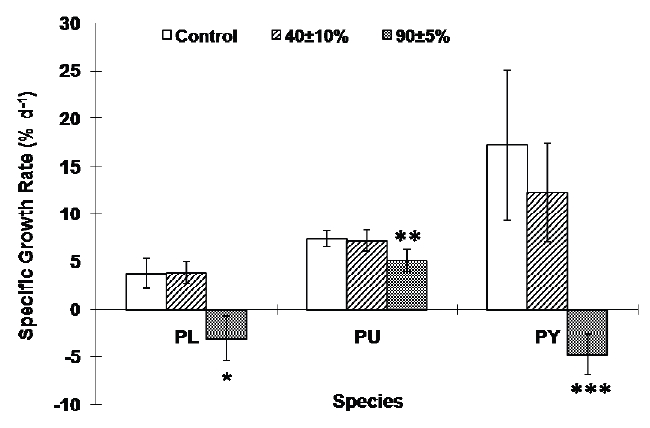
Desiccation significantly influenced the growth rate of all three species. The sublittoral species P. yezoensis and the lower eulittoral species P. leucosticta lost weight under severe desiccation stress while the growth rate at moderate desiccation conditions was not significantly different from the control which had continually submerged tissues (Fig. 3). The growth rate of P. umbilicalis also decreased at the severe desiccation condition, and it was about 70% of the continually submerged tissues (p < 0.001, Fig. 3).
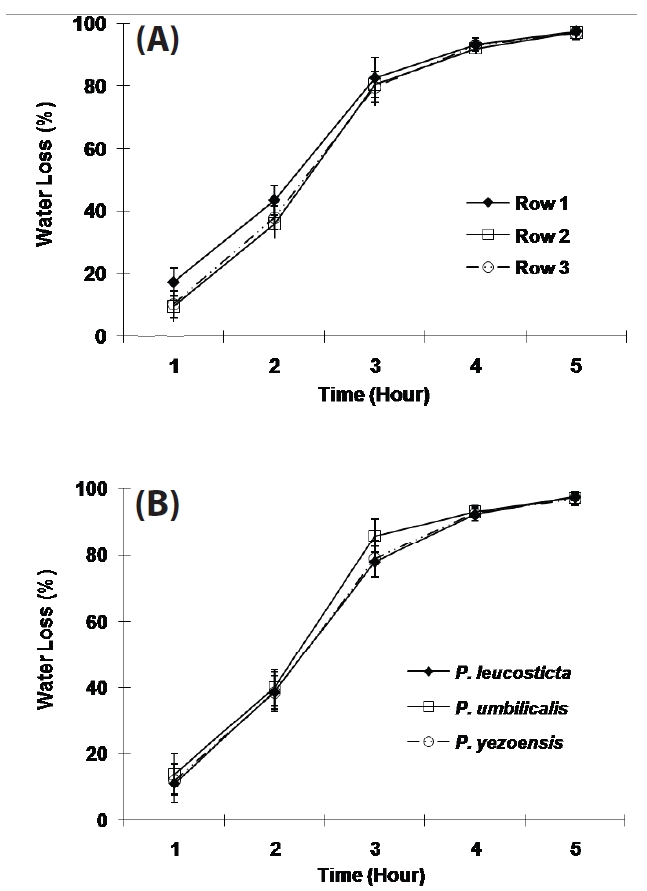
The variation of the WL in each compartment was up to 20% for the first 3 hours of exposure, and then the variation decreased to less than 10% as exposure time increased. The WL of samples in the front row was significantly higher than the other two rows for 2 hours after exposure (p < 0.001, Fig. 4A).
The WL was not significantly different in different Porphyra species except for the period of three hours exposure (p < 0.001, Fig. 4B). All three Porphyra species showed approximately 40% WL in two hours and approximately 90% WL in four hours in the greenhouse on cloudless and partly cloudy days (Fig. 4B).
We found that Porphyra species grew on average 17% (P. yezoensis), 4% (P. leucosticta) and 7.5% (P. umbilicalis) daily under continually submerged condition. Recently, Carmona et al. (2006) cultured seven Porphyra species, including the species used in this study, at various nitrogen concentrations in 1 L culture chamber. Their culture conditions were analogous to the controls in this study with the exception that Porphyra in this study grew in a greenhouse under ambient light condition. They found similar growth rates of the same species at 25 and 75 μM of nitrogen which were 6-10% (P. umbilicalis), 1-5% (P. leucosticta) and 4-15% (P. yezoensis) respectively. These results suggest that Porphyra species can grow very well in this tide simulating apparatus, and data obtained by using this apparatus can be reliable.
Desiccation was more stressful to the lower eulittoral species P. leucosticta and the sublittoral species P. yezoensis than it was to the upper eulittoral species, P. umbilicalis. Both P. leucosticta and P. yezoensis could not grow after severe desiccation stresses while P. umbilicalis still grew under severe desiccation stress to approximately 70% of continually submerged tissues. Species living at one elevation on the shore may be better adapted to live, grow, and reproduce in that environment than organisms that grow elsewhere (Luning 1990, Kim et al. 2008). These results suggest that the upper eulittoral species P. umbilicalis may be adapted to the severe desiccation stress unlike species from lower eulittoral and sublittoral zones. Therefore, these results indicate that desiccation stress should be a critical factor that will determine the upper distribution limits of seaweeds (Thomas et al. 1987, Davison and Pearson 1996, Beach and Smith 1997).
The tide simulating apparatus developed in this study is specifically designed for macroalgae or other sessile organisms. This apparatus can allow varying exposure times, simulating different vertical elevations within the eulittoral zone. This machine can also be utilized to study the effects of exposure to any air/gas mixture (c.i. varying levels of CO2) under controlled conditions. Unlike eulittoral habitats, with this apparatus, water is stationary, and the “shore” itself moves up and down simulating tidal cycles. This type of tide simulating apparatus can be easily built and is cost effective.
The tide simulating apparatuses from the 1970s were not broadly utilized although there were potential applications for the study of eulittoral organisms because of their scale of operation and lack of replication. The apparatus developed in this study consists of 18 independent culture tanks (3 blocks × 6 culture tanks), and each tank can contain up to 2.5 L of growth medium. Therefore, this apparatus allows for independent replicates and can be utilized for large macroalgal thalli. In other tide simulating apparatuses, there was also an issue of balance and stability. In this apparatus those issues are resolved by using a stainless steel braided cable connected to each corner of the plate, so that the whole plate can be raised or lowered while remaining level.
In the preliminary tests with three Porphyra species, WL in each tank varied, 15-50% in 2 hours, 50-80% in 3 hours and 75-95% in 4 hours on partly sunny days. The WL of samples in the front row was higher than the other rows, though this difference diminished with increasing desiccation. This result may be due to the south facing position of the apparatus in the greenhouse. Samples in the front row may have received more light during exposure than samples in other two rows. Weather conditions also affected the WL, since this apparatus was not installed in a temperature and humidity controlled environment. To maintain a similar WL in each row, fans were used. Variation was retained down to 20% for the first 3 hours and then decreased to less than 10% as exposure time increased.
The entire apparatus is rather small in size (100 cm, length, × 50 cm, width, × 60 cm, height) and therefore, is easily disassembled and movable to any indoor laboratory, greenhouse, etc. If this apparatus was installed in an environmentally controlled chamber, the variation of WL would even more decrease. In an environmentally controlled chamber, one can test the emersion effects at various environmental conditions such as temperature, humidity, nutrient, salinity, photoperiod, light (quantity and quality), etc. Less physical efforts by human will be required for emersion, and each condition will provide a wide range of reproducibility. This apparatus also offers considerable flexibility in terms of design. We designed this apparatus to culture particularly a small or medium sized thalli (< 15 cm in length; e.g., Porphyra, Ulva, Chondrus, etc.) since many eulittoral seaweeds are within this range. However, by simply changing the size of culture tank (either increasing the height of cylinder or/and using a different diameter of cylindrical Plexiglas), larger/taller thalli including fucoids (e.g., Fucus, Ascophyllum, etc.) can be cultivated.
In conclusion, the tide simulating apparatus developed in the present study has solved many of the problems affecting similar apparatuses in earlier years. The volume of the apparatus is scaled up, so macroalgal thalli can be cultured. The balance of the apparatus when plants are exposed is not problematic in this apparatus. This apparatus includes independent tanks to satisfy the requirements for statistical replication. The tide simulating apparatus also exhibits improvements in cost, space efficiency, simplicity of operation and application for thalli of other benthic marine macroalgae. We suggest that this apparatus may be utilized for wider applications in the study of emersion on other eulittoral organisms with some modifications including sessile eulittoral mollusks or crustaceans. This apparatus may also be used for seaweed aquaculture purposes since exposing seaweeds regularly to tide cycles can eliminate epiphytes (Sahoo and Yarish 2005) and enable the study on emersion on the chemical constituents in the thalli.