In Korea,
[Table 1.] Information of samples used in molecular analysis
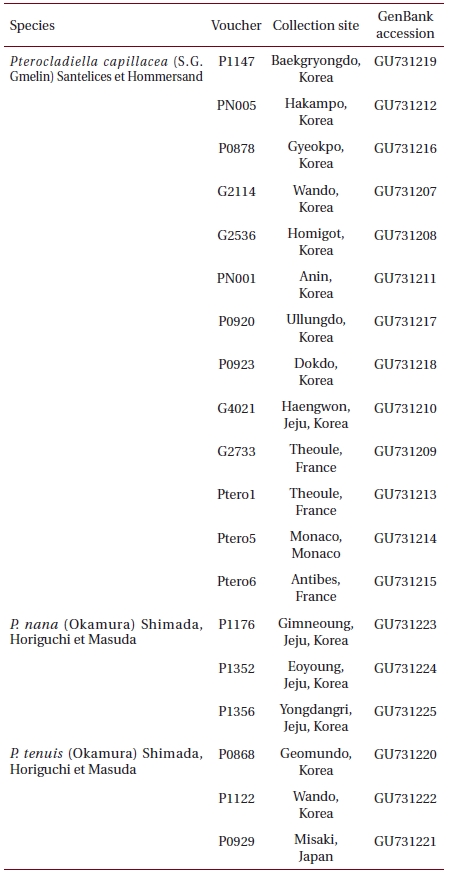
Information of samples used in molecular analysis
In the present study, we investigated the morphology of Pterocladiella through extensive collections along the coasts of Korea, observing details of morphology and analyzing plastid rbcL from representative collections of the species. Since the type locality of P. capillacea is the Mediterranean Sea (Womersley 1994), we included samples collected in Nice, France in the present study. The sequences from P. tenuis and P. nana collected in their type localities are also available (Shimada et al. 2000). Published sequences of other species of Pterocladiella (Tronchin and Freshwater 2007) allowed us to directly compare our results with others from the genus as a whole and with putative relatives.
Field observations and collections of
>
DNA extraction and analysis of rbcL region
Intotal, 19 specimens were available for molecular analysis (Table 1). Genomic DNA was extracted from approximately 5 mg of dried thalli ground in liquid nitrogen with the Invisorb Spin Plant Mini Kit (Invitek, Berlin, Germany), according to the manufacturer’s instructions: extracts were dissolved in 200 μL of distilled water. The extracted DNA was stored at -20°C and used to amplify rbcL. Voucher specimens used for molecular systematics were deposited in the herbarium of CNUK.
For amplification and sequencing reaction of
In accordance with our objective to reexamine the three Pterocladiella species, we only included species of this genus for reconstructing rbcL tree. Maximum parsimony (MP) trees were constructed for each dataset with PAUP*4.0b.10 (Swofford 2002) using a heuristic search algorithm with the following settings: 1,000 random sequence additions, tree bisection-reconnection branch swapping, MulTrees, all characters unordered and unweighted, and branches with a maximum length of zero collapsed to yield polytomies. Bootstrap values for the resulting nodes were assessed using 1,000 bootstrapping replicates with ten random sequence additions. The maximum likelihood analyses were implemented using the online version of RAxML program (Stamatakis 2006) and the GTR + Γ evolutionary model. We used 200 independent tree inferences, using -# option with default -I (automatically optimized SPR rearrangement) and -c (25 distinct rate categories) options of the program to identify the best tree. To generate bootstrap values for these phylogenies, we used RAxML with the same settings and 1,000 replications. Bayesian (BA) analyses were conducted with MrBayes v3.1 software (Ronquist and Huelsenbeck 2003) using the Markov chain Monte Carlo method (MC2) with the GTR + Γ + I model. Three million generations in two independent runs were performed with four chains and trees were sampled every 100th generation. The burn-in period was identified graphically by tracking likelihoods at each generation to determine whether the likelihood values had reached a plateau; the burn-in periods were 10,250 for
>
Pterocladiella capillace (S.G. Gmelin) Santelices et Hommersand
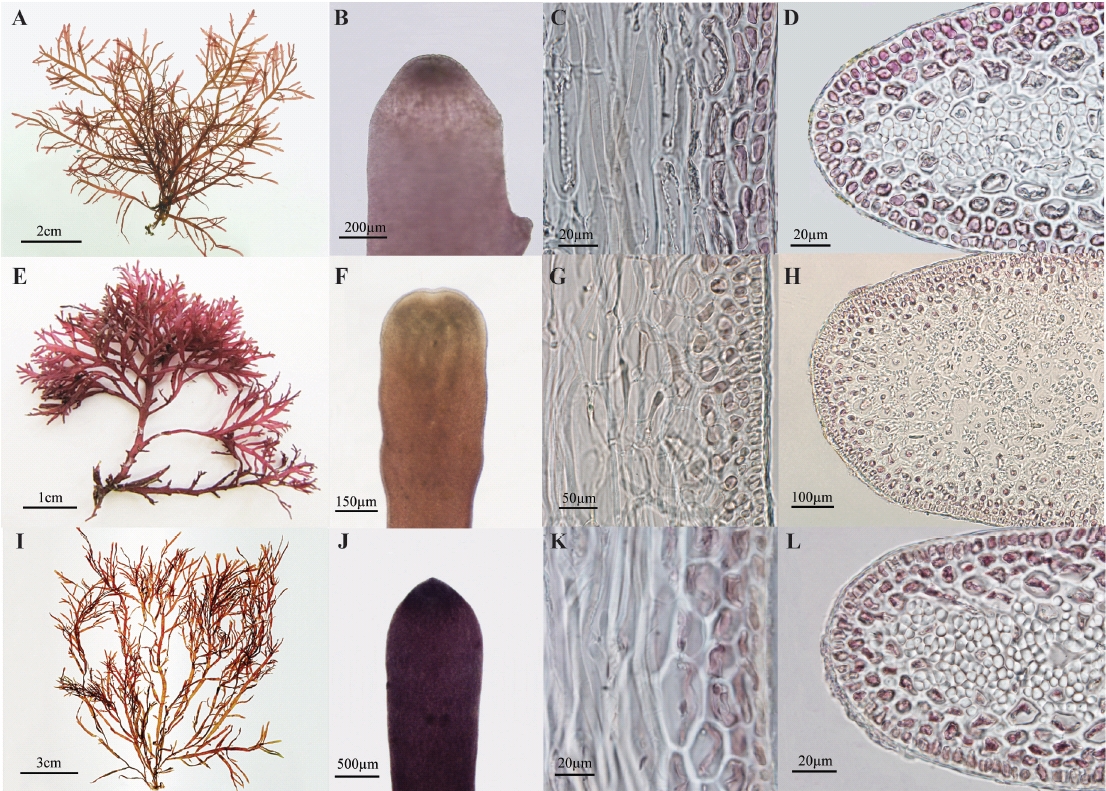
Thalli are up to 10 cm tall arising from a stoloniferous holdfast, showing a christmas tree shape outline (Fig. 1A). Erect axes are cylindrical without branches at base and become flattened with branches at a single plane. Branching is regularly 3-4 times pinnate to opposite, up to four-orders. The first-order branches have a basal constriction and emarginate or exhibit blunt apices (Fig. 1B). They are regularly pinnate. The second-order branches have simple third order-branches. Apical cells are dome-shaped and occasionally protuberant (projecting). The inner cortical cells are larger, angular, and arranged in rows, making a cortical layer of 2-3 cells (Fig. 1C). In transverse sections of the middle part of uprights, the outer cortical cells are isodiametric (Fig. 1D). Medullary cells are colorless without rhodoplasts. Rhizoidal filaments usually originate from third-order cells in cortical layer as a protuberance. They are thick-walled unicellular filamentous cells, refractive, with a tiny lumen. Rhizoidal filaments are often more congested in medullary layers than in cortical layers.
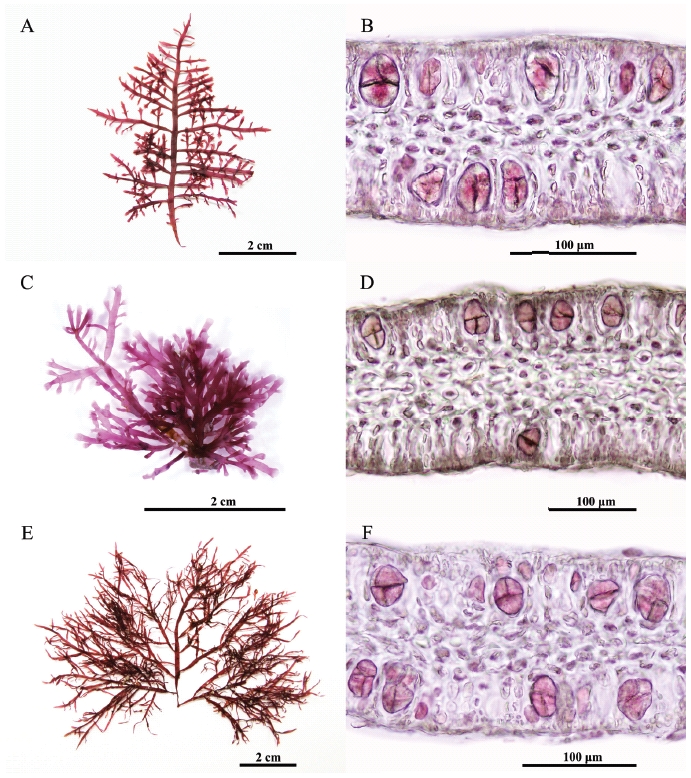
Tetrasporangial sori occur in terminal branch swellings and become gradually elongated. Tetraspora-ngia are cruciately to irregularly divided (Figs 2A & B). Female and male thalli were not found in our collections.
Korean name: Gaewoomu (개우두)
Selected specimens: Junghwadong, Baekryongdo (SY Kim, 21 vi 2008); Geokpo, Buan (IK Hwang, 16 xi 2005); Anin, Kangneung (SM Boo, 14 x 2007); Hakampo, Taean (SM Boo, 21 v 2007); Jeongdori, Wando (SM Boo, 3 xi 2006); Homigot, Pohang (IS Hong, 24 ii 2007); Haengwon, Jeju (IK Hawng, 17 vi 2008); Ullungdo (IK Hawng, 22 vi 2007); Gamchusa, Donghae (SM Boo, 5 vii 2008); Muchangpo, Seochun (IK Hawng, 30 vii 2008).
Thalli grow abundantly on a diverse range of other algae, boulders and bedrocks in the lower intertidal and upper subtidal zones. Thalli were collected in June, August, October, and December 2004 to 2006, off the coast at Gampo, Korea. Young thalli began to appear in June, and became reproductive with tetrasporangial sori from August through October when thalli achieved the largest size. In January, thalli were very small and scarce.
>
Pterocladiella nana (Okamura) Shimada, Horiguchi & Masuda
Thalli are up to 5 cm tall arising from a stoloniferous holdfast, having a corymbose outline (Fig. 1E). Erect axes are cylindrical without branches at base and become flattened with branches at a single plane. Branching is regularly 2-3 times pinnate to opposite. The first-order branches have a basal constriction, and emarginate or exhibit blunt apices (Fig. 1F). They are regularly pinnate. The second-order branches have simple third order-branches. Apical cells are dome-shaped and occasionally protuberant (projecting). The inner cortical cells are larger, angular, and arranged in rows, making a cortical layer of 2-3 cells (Fig. 1G). In transverse sections of the middle part of uprights, the outer cortical cells are isodiametric (Fig. 1H). Medullar cells are colorless without rhodoplasts. Rhizoidal filaments usually originate from third-order cells in cortical layer as a protuberance. They are later thick-walled unicellular filamentous cells, refractive, having a tiny lumen in the midst. Rhizoidal filaments are often more congested in medullar layers than in cortical layers.
Tetrasporangia are irregularly disposed at the apices of axes and branches, and cruciately divided (Figs 2C & D). Cystocarpic and spermatangial thalli were not found in the present study.
>
Korean name: Jageun-Gaewoomu (작은개우무)
Selected specimens: Gimnyeong, Jeju (SY Kim, 6 v 2008, CNU C007022-7; IS Hong, 21 vii 2008); Yongdangri, Jeju (IS Hong, 21 vi and 22 vii 2008); Doduri, Jeju (SM Boo, 13 iii 2009); Eoyoung, Jeju (IS Hong, 16 ix 2008).
>
Pterocladiella tenuis (Okamura) Shimada, Horiguchi et Masuda
Thalli are up to 19 cm tall (average 10.8 cm) arising from a stoloniferous holdfast, having a fan shape outline (Fig. 1I). Erect axes are cylindrical without branches at base and become flattened with branches at a single plane. Branching is 2-3 times pinnate to opposite. The first-order branches have a basal constriction, and emarginate or exhibit blunt apices (Fig. 1J). They are regularly pinnate. The second-order branches have simple third order-branches. Apical cells are dome-shaped and occasionally protuberant (projecting). The inner cortical cells are larger, angular and arranged in rows, making a cortical layer of 2-3 cells (Fig. 1K). In transverse sections of the middle part of uprights, the outer cortical cells are isodiametric (Fig. 1L). Medullary cells are large and colorless. Rhizoidal filaments usually originate from third-order cells in cortical layer as a protuberance. They are thick-walled unicellular filamentous cells, refractive, with a tiny lumen. Rhizoidal filaments are often more congested in cortical layers than in medullar layers.
Tetrasporangial sori are formed roundish patches in the ultimate branchlets, and become gradually elongated (Figs 2E & F). Tetrasporangia are cruciately divided, 48-52 μm long and 20-36 μm wide. Female and male thalli were not found in our collections.
Korean name: Keun-Gaewoomu (큰개우무)
Specimens examined: Jedo, Wando (TO Cho, 12 vi 1999, CNU C006002, C006051); Geomundo, Yeosu (SM Boo, 25 ix 2005, CNU C007001-5); Jeongdori, Wando (IK Hwang, 8 vi 2007, CNU C007006-9); Mangsukri, Wando (IK Hwang, 19 ix 2007, CNU C007010-2); Hado, Jeju (SM Boo, 20 x 2007, CNU C007013-6); Hyeongje-seom, Goheung (TO Seo, 1 v 2008, CNU C007017-20); Nando, Jeju (IK Hwang, 12 ix 2005).
Thalli grow on boulders and bedrocks in the subtidal zones on the southern coast of Korea. Thalli were collected in October 2006 in Geomundo, in July 2007 at Mangsukri in Wando, and in December at Hado in Jeju. Young thalli began to appear in June, and became mature with tetrasporangial sori from August through October 2006, when plants achieved largest size. In January 2007, thalli were very small and scarce.
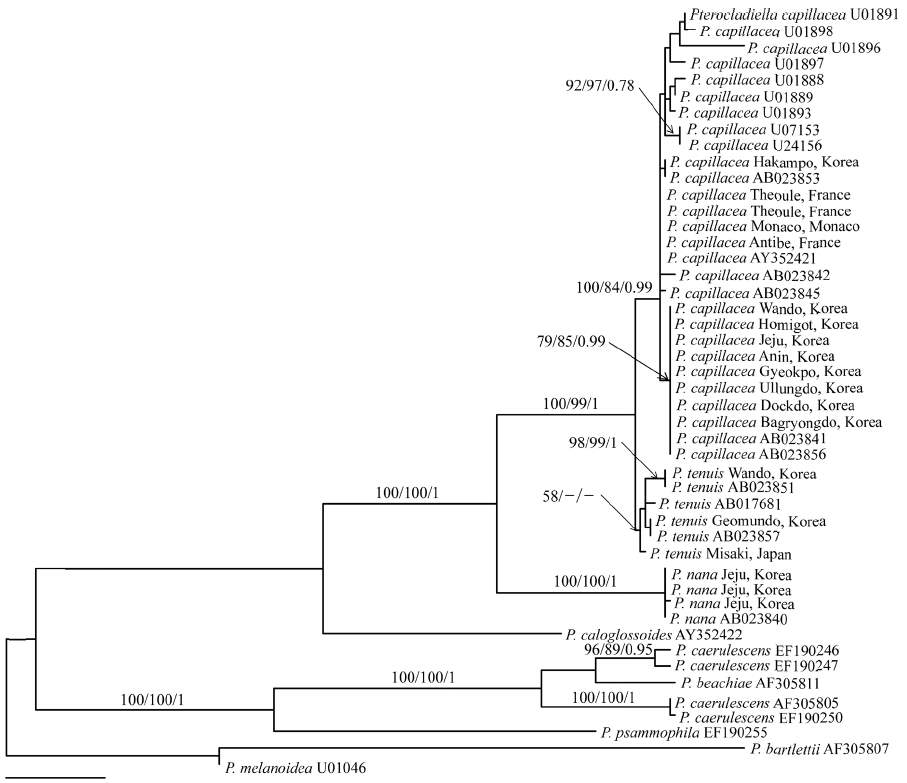
Twenty-eight rbcL sequences of P. capillacea including four P. nana and six P. tenuis, were aligned using a 1,353 nucleotide portion of the rbcL gene. Variable sites occurred at 306 positions (22.6%), and among them, 214 positions (15.8%) were parsimoniously informative. Sequences of P. capillacea specimens from all of Korean localities (except one place; Hakampo), were identical with those from Japan. They differed by 2 bp (0.15%) from those of France, which are identical to those from New Zealand. However, rbcL gene was highly variable, including 12 haplotypes within the species. P. tenuis differed by 7-27 bp (0.5-2%) from P. capillacea. In the phylogenetic tree (Fig. 3), specimens of P. capillacea from Korea, Japan, France, Spain, Ireland, Venezuela, USA, Australia, and New Zealand produced monophyly with strong support values (100% for ML, 84% for MP, and 0.99 for BA). In all MP, ML, and BA analyses, P. capillacea and P. tenuis formed a sister relationship with strong support and P. nana was basal to the clade.
Through detailed morphological observations and analysis of rbcL sequences, we demonstrated marked difference among P. terocladiella capillacea, P. nana, and P. tenuis, unraveling the occurrence of these three species in Korea. P. terocladiella capillacea is distinguished by its thalli showing a christmas tree shape, regular and pinnate first-order branches, and abundant, slender second-order branches with short intervals of branching. Internal structure and reproduction of P. capillacea is well illustrated by Dixon (1958), Fan (1961), and Akatsuka (1986). This species is abundant from summer to fall in the lower intertidal and subtidal zones along the coast of Korea. The rbcL sequences from Korea and France and previously published data from Ireland, Italy, Japan, and Australia (Freshwater et al. 1995, Shimada et al. 2000) consistently reveal that all specimens of P. capillacea were monophyletic with a strong bootstrap support. This result supports the cosmopolitan distribution of P. capillacea that spans geographical barriers between Korea, northeast Asia, Europe, Oceania, and America. Given that 12 rbcL haplotypes were found in P. capillacea, fast-evolved genes such as mitochondrial cox1 need to be analyzed to order to explain the genetic diversity of this species. It will also be interesting if sampling could be extended to the Indian Ocean (Silva et al. 1996), Caribbean islands (Richardson 1975), north and south America (Stewart 1968, Ramirez and Santelices 1991), and Africa (Lawson and John 1987), where P. capillacea occurs.
Although Santelices (1999) treated P. nana Okamura (1932) as a synonym of P. capillacea, Shimada et al. (2000) reinstated P. nana based on rbcL sequences and designated a specimen as lectotype of this species, which was collected in Koshiki Islands, Kagoshima Prefecture in July 1919. Our molecular results show that three sequences from Jeju were identical with that from Japan, and clearly separated from P. capillacea, as seen by Shimada et al. (2000). Thalli from Korea agree well with the lectotype specimen, deposited in SAP in Sapporo (Shimada et al. 2000). P. terocladiella nana is distinguished by the small size of its thalli (up to 5 cm), up to three-order branches, evenly distributed rhizoidal cells in cortical and medullar layers, and tetrasporangial sori in ultimate branches. Although Kim (2001) found a single thallus of this species in Jeju, we found P. nana in three different locations and in different seasons on the same Island. Since it also occurs in Fiji, Philippines, and South Africa (Silva et al. 1996, Shimada et al. 2000), P. nana is considered as a warm-water species.
Pterocladiella tenuis occurs in the lower intertidal to subtidal zones on the southern coast of Korea. The species is characterized by large, thin and flat thalli with irregular branches, and first-order branches with rarely second-order branches. In rbcL tree, the Korean specimens clustered with specimens from Japan, which included specimens from the type locality (Shimada et al. 2000). However, P. tenuis has two forms depending on depth; thalli in deep waters have distantly and loosely arranged branches, while thalli occurring intertidally are closely branched (Okamura 1934). The reexamination of Okamura’s intertidal form will show whether the ecologically and morphologically distinguished forms are genealogical lineages or different species. Compared to cosmopolitan distribution of P. capillacea, P. tenuis is limited to Korea and Japan (Shimada et al. 2000, the present study).
Although gametophytes and tetrasporophytes are isomorphic in the order Gelidiales, tetrasporangial thalli generally predominate over gametophytic thalli in the field (e.g., Akatsuka 1986, Neto 2000, Serviere-Zaragoza and Scrosati 2002). For example, gametophytes were found only in January, with even lower percentages (0.15-0.1%) in Baja California, despite the overall predominance of tetrasporangial thalli (Servierse-Zaragoza and Scrosati 2002). Neto (2000) found only one cystocarpic thallus in April, despite the occurrence of many tetrasporangial thalli in summer and winter, in the Azores Islands. In Korea, Sohn and Kang (1978) found no gametophytes and Kim (2001) found female thalli in August in Haengwon of Jeju, despite his extensive collections along the coasts of Korea. In the present study, tetrasporangial thalli commonly occurred in each of the Pterocladiella species, but no female and male gametophytes were found. It may be a general phenomenon that most Korean populations of Pterocladiella species repeat tetrasporangial stages. However, ecological studies on Korean species are necessary to highlight factors affecting the predominance of tetrasporangial stage over gametangial stages.
In conclusion, our morphological and molecular investigations confirmed the occurrence of P. capillacea, P. nana, and P. tenuis in Korea. Although P. densa and P. robusta have been reported in Korea, both species are treated as synonym of P. capillacea (Stewart 1968, Shimada et al. 2000). Because both P. tenuis and P. nana may be confused with P. capillacea, the diversity of the genus should be further evaluated based on careful field collections.