In most eukaryotic systems the process whereby one cell becomes two (or many) occurs in two distinct stages: mitosis or nuclear division, and then cytokinesis or cell division. In some organisms such as species of multinucleate green and red algae (e.g., Pickett-Heaps 1975; Garbary and McDonald 1995) and some fungi (e.g., Carlile et al. 2001), mitosis may occur in the absence of cytokinesis, resulting in multinucleate cells. In the brown algae, however, where vegetative cells are uninucleate (e.g., van den Hoek et al. 1995; Graham and Wilcox 2000), mitosis is typically followed by cytokinesis. Cell division has been described for a number of brown algae (e.g., La Claire 1982; Katsaros et al. 1983, 2006; Motomura and Sakai 1985; Bisgrove et al. 2003; Bisgrove and Kropf 2004), and the process is considered to be relatively uniform (van den Hoek et al. 1995). Like other eukaryotic systems, cell division in brown algae is assumed to follow the classic model of mitosis followed by cytokinesis. However, unlike angiosperms, cytokinesis takes place in binucleate cells, because it occurs after mitosis is completed (Katsaros et al. 2006). In addition, there are no cortical microtubules, and therefore no preprophase band to mark the future site of cytokinesis, nor is there a phragmoplast (Katsaros et al. 2006). Bisgrove et al. (2003) suggested that the division plane in a fucoid alga was established at the boundaries of the cytoplasts.
Here we describe a novel developmental pattern in the fucoid seaweed, Ascophyllum nodosum, in which cytokinesis occurs in the absence of mitosis. Such a phenomenon represents a previously undescribed form of programmed cell death in photosynthetic organisms, as the resulting cells have no capacity for growth or proliferation and are shed from the producing thallus soon after formation. Autophagic and apoptosis-like modes of programmed cell death are the two main types of programmed cell death in plants (Love et al. 2008; Reape et al. 2008); these may occur in nature during development, for example in tissue remodeling (Gunawardena 2008) or in response to environmental factors (Love et al. 2008). In either case, the entire contents of a normal cell, including the nucleus, is degraded. Programmed cell death is a common feature of many plant and photosynthetic systems (e.g., Gunawardena 2008; Reape et al. 2008) including some algae (Garbary and Clarke 2001).
The process we describe is associated with the outer cell layer or meristoderm cells (Fritsch 1945) of Ascophyllum nodosum (hereafter Ascophyllum), and the resulting anucleate cells (epidermis) are shed as a sheet of cell walls and the resulting cytoplasts or their remnants. Epidermal shedding has long been known in fucoids; however, neither the cytology of these cells nor details of their development have been characterized. Filion-Myklebust and Norton (1981) initially described epidermal shedding in Ascophyllum. They showed that layers of material were shed from the Ascophyllum thallus at regular intervals. Epidermal shedding was considered an adaptation to remove fouling organisms from the surface of the thallus, and was also demonstrated in Halidrys siliquosa (Moss 1982, 1984; Russell and Veltkamp 1984). This process may characterize many long-lived fucoid algae (Moss 1984). Here we provide cytological details of the process to characterize a new form of programmed cell death in multicellular, photosynthetic eukaryotes.
Fronds of Ascophylllum were collected from the intertidal zone of Antigonish Harbour (45°40’51” N, 61°52’5”W) and Tor Bay (45°10’57”N, 61°21’13”W), on the northeastern mainland of Nova Scotia between May and August, 2007. For some observations fronds were fixed in the field (see below), whereas for others, fronds were transferred to the laboratory and maintained at 15°C in 750 L tanks of circulating seawater (salinity of 30 ppt) until use. Tanks were illuminated with cool-white fluorescent bulbs with daylength set to outdoor daynight cycles (ca. 15 h light, 9 h dark). The floating thalli were exposed to about 20 μmol photons m-2 s-1 of photosynthetically active radiation.
Six large fronds were tagged in Antigonish Harbour from which single apical segments from the current year’s growth were removed at 4 hr intervals over a 24 hr period on 5-6 June 2007. Frond segments were immediately fixed in 95% ethanol: glacial acetic acid (3:1, v/v) for 24 h. Specimens were transferred to 70% ethanol and stored at 4°C. Frond segments were embedded in Cryo- Gel (SPI Supplies, Toronto, Ontario, Canada) and frozen at -80°C prior to sectioning with a Cryo-Cut Model 845 microtome (American Optical Corp., Southbridge, MA, USA). The resulting transverse 15 μm sections were stained with DAPI (Sigma Chemical Co., St. Louis, MO, USA) as described in Garbary and Clarke (2002), and observed by fluorescence microscopy with UV excitation using a Zeiss Photomicroscope III (Zeiss, Oberkochen, Germany) (McDonald et al. 1993). Some hand sections were observed unstained or following staining with 10 μM FB28 (Sigma Chemical Co.) (Garbary and Belliveau 1990).
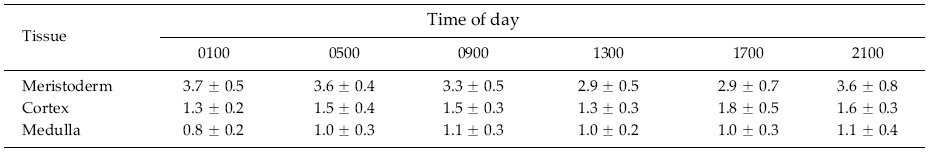
The mitotic index was determined using a protocol modified from Kapraun and Boone (1987). Sections were scanned at 800x magnification until a mitotically active cell was identified. The total number of cells in the field and the number of mitotically active cells were counted. Multiple fields of view were examined for each tissuetime combination until 500 cells were characterized in each of the meristoderm, cortical and medullary cell layers (see results for details of anatomy).
Photomicroscopy was carried out using a Microphot- FXA microscope (Nikon, Tokyo, Japan) equipped with a SPOT model 1.4.0 digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA).
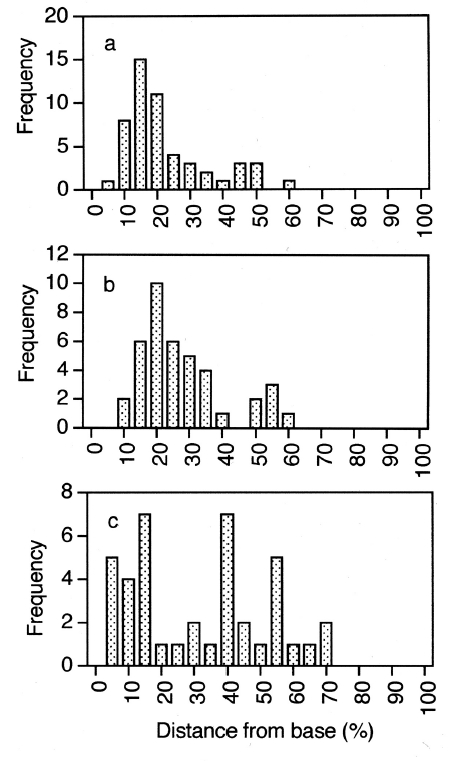
DAPI-stained transverse sections were used to determine: i) meristoderm protoplast length from basal to apical ends, and ii) the distance from the protoplast base to the midpoint of nuclei in each cell. This was carried out in uninucleate cells (n = 50), and binucleate cells (i.e., post-mitotic cells that have not yet undergone cytokinesis) with nuclei horizontally or vertically oriented relative to thallus surface (n = 40 nuclei).
Portions of freshly collected thalli were fixed in a mixture of 5% glutaraldehyde, 3% sucrose, and 0.1 M phosphate buffer (pH = 7.8) for 24 h at 4<>;C. Material was dehydrated in graded steps of ethanol over 24 h ending with several changes in 100% ethanol. Thallus fragments were mounted on stubs and coated with gold using a Polaron SC502 sputter coater (VG Microtech, Uckfield, East Sussex, UK). A JEOL JSM-5300 SEM (Jeol, Tokyo, Japan) was used at 5-25 kV. Digital images were captured using a JEOL DSG1 frame store system.
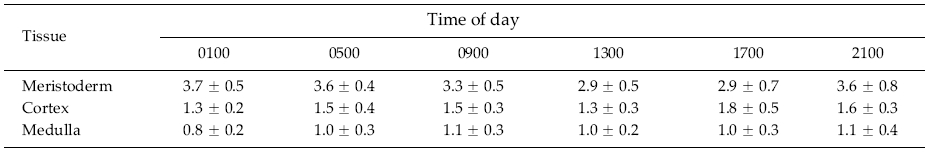
The traditional perspective of vegetative frond anatomy in fucoids is that there is an outer meristoderm layer of relatively small, palisade-like cells, an extensive region of cortical tissue comprising larger, rounded to polygonal cells, and a central region of longitudinally-elongated cells comprising medullary tissue (Fritsch 1945). Filion- Myklebust and Norton (1981) showed that there was an additional cell layer formed by the meristoderm that was shed at regular intervals. Here we will refer to this layer of cells as the epidermis.
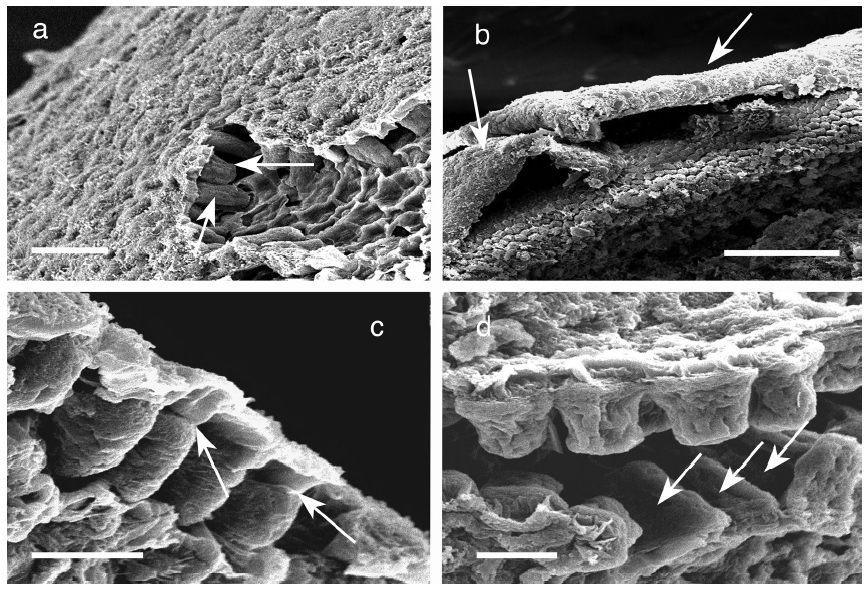
Meristoderm cells are slightly elongated and form a palisade-like layer, with individual cells 50-100 μm tall and 30-40 μm wide (Figs 1a-d). Chloroplasts are always at the base of the cells (Xu et al. 2008) and autofluorescence of chlorophyll is never in the upper portions of these cells (Fig. 2). Nuclei are at the base of meristoderm cells. Following cell elongation, a cell plate develops between constrictions in the tangential cell walls about 60-75% of the cell length from the base. This leads to the formation of a small epidermal cell. Since cytokinesis is more-or-less synchronous across large portions of the thallus surface, a distinct layer of epidermal cells is formed. This layer detaches from the underlying meristoderm and is shed from the thallus. After shedding, the newly exposed surface may have an extremely regular, tessellated surface, or retain remnants of the cell walls of the shed epidermal layer. The latter is visualized as surface ridges that are sometimes present on the epidermis (Fig. 1d).
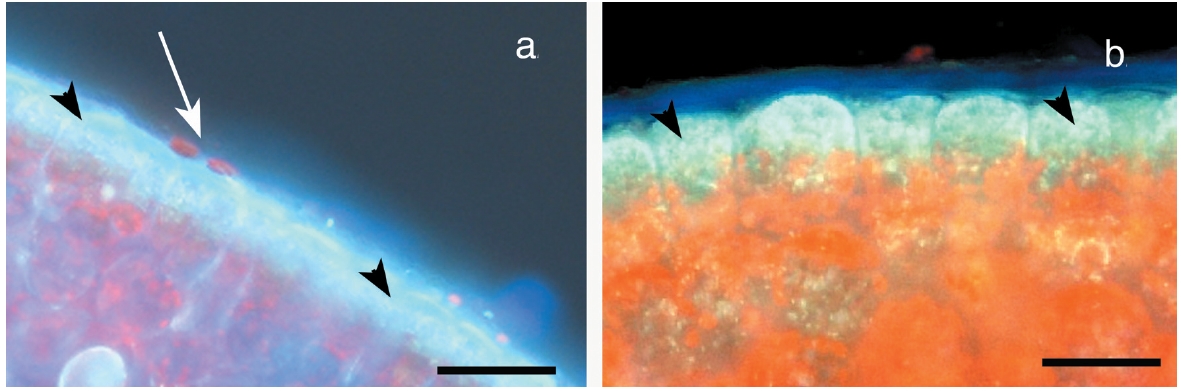
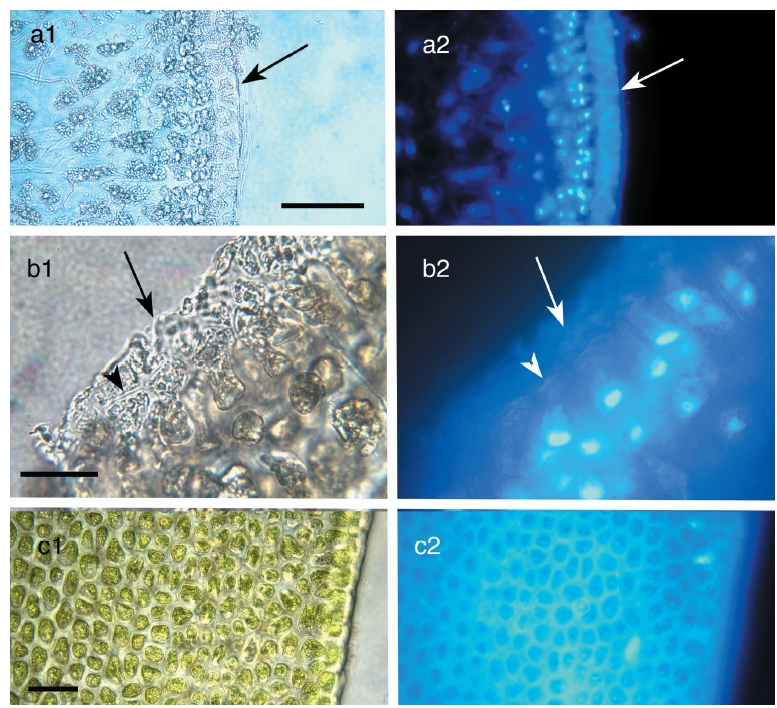
The primary observation of this research is that the epidermal cells are anucleate during all stages of development. DAPI-staining of transverse sections revealed a translucent epidermal layer in which there are no nuclei and a meristoderm layer in which the nuclei are prominent when viewed with UV excitation. Thus DAPI- staining never reveals nuclei in the shed epidermal cells at any stage of their development (Fig. 3). Cell walls of the epidermal cells fluoresce weakly due to trapping of some DAPI in the walls. Isolated sheets of epidermal cells appear clear to greenish when viewed by brightfield microscopy, with the greenish colour induced by the microwave fixation procedure (Fig. 3). This colour is attributed to phenolic components rather than photosynthetic pigments, because the basal location of chloroplasts in meristoderm cells precludes their incorporation into anucleate epidermal cells. Chlorophyll fluorescence (not shown) is absent in epidermal cells, but prominent in meristoderm and cortical cells.
This experiment was used to determine if a clear diurnal pattern of mitosis was present. If this was the case, then subsequent field collections would be made when maximal mitotic activity was occurring. These observations were made so as to eliminate the possibility that there was a specific time of day when mitosis might be occurring in meristoderm cells, that was followed by rapid development of the cell plate and subsequent nuclear degradation. Thus we ensured that the shed epidermis was not being formed during such a period of mitotic activity by normal mitoses followed by cytokinesis and subsequent nuclear degradations, such as occurs in typical cases of programmed cell death.
There was a considerable range of mitotic divisions in frond/time combinations from 0.6% (medullary cells at several time periods) to almost 5% (meristoderm cells at 2100 h). The evaluation of mitoses over a 24 h period in the meristoderm, cortical and medullary cells of Ascophyllum revealed two major patterns. With regard to diurnal timing, there was little apparent change in mitotic activity (Table 1), and the repeated measures ANOVA showed no significant difference (p >; 0.05) among any of the time periods. The percentage of mitoses in meristoderm cells varied from 2.92 ± 0.69% to 3.73 ± 0.47% (mean ± SD). The corresponding values for cortical cells were 1.26 ± 0.24% to 1.76 ± 0.49%, while values for medullary cells varied from 0.83 + 0.2% to 1.07 ± 0.37%. The three tissues showed significant differences in the mitotic index, with values of 3.42 ± 0.30%, 1.50 ± 0.19% and 0.98 ± 0.09% in meristoderm, cortical and medullary cells, respectively. The increase in mitotic activity in meristoderm cells relative to cortical cells is explained by the fact that cross sectional area of fronds undergoes a substantive increase (i.e., 65%) between the growth in the current year and the previous year, with new cortical cells being formed from the meristoderm. The difference between cortical and medullary cells is at least partly explained by the fact that medullary cells are longitudinally elongated, and the nuclei in these cells are less likely to be observed in transverse sections.
Observations using hand-cut sections and evaluation of previously carried out transmission electron microscopy (Xu et al. 2008) showed that nuclei and chloroplasts in meristoderm cells were predominantly located in the basal portion of cells. Quantitative evaluation of nuclear position in uninucleate and post-mitotic, binucleate cells is shown in Fig. 4. In uninucleate cells (Fig. 3a) the mean midpoint of nuclear position was 18.7% of the distance from the cell base to the cell apex. Nuclei in the bottom 20% of meristoderm cells accounted for two thirds of all nuclei. Furthermore, only a single nucleus was positioned above the mid point of the cell. Given that the epidermal cells comprise a maximum of 30% of what was the upper part of the meristoderm cell prior to cytokinesis, none of these nuclei were positioned so as to be incorporated into the developing epidermal cells.
In binucleate cells, nuclei pairs may be oriented moreor- less side by side either parallel to or perpendicular to the thallus surface. In the former case (Fig. 4b), nuclear position was apparently bimodal, with most nuclei located in the lower 15-20% of meristoderm cell length as measured from the basal surface, but with a smaller number located midway at 50-55% of cell length. No nuclei were ever observed in the upper 40% of cell length. The six nuclei in the upper peak (Fig. 4b) comprised only 15% of the nuclei whose position was determined. When nuclei of binucleate cells were vertically oriented, nuclei were much more widely distributed (Fig. 4c); however, most nuclei were in the lower half of cells. Thus, nuclei were never observed in the upper 25% of meristoderm cells where they might be included in the epidermal cell formed after cytokinesis.
This paper describes two unusual cytological processes processes in plants and multicellular algae. The first process is the regular occurrence of cytokinesis without cells first having undergone mitosis, making it a fundamental developmental phenomenon in this species. The second process is the shedding of cells in a distinct cell layer. The former process is unique, while the latter process is analogous with other photosynthetic organisms (e.g., epidermal shedding in calcified red algae (see below).
The plant kingdom is notable for many variations on the standard cell cycle, such as S-phase DNA synthesis alone, or the complete cell cycle without DNA synthesis, or mitoses without cytokinesis. This flexibility in cell cycle control may help these immobile organisms adapt to varying environmental conditions (De Veylder et al. 2007). In brown algae, a variety of cytological anomalies have been reported. For example, following meiosis in the sporangia of the kelp Laminaria saccharina, the four resulting nuclei already have twice the nuclear DNA levels as before meiosis. In addition, these nuclei then undergo at least three rounds of mitoses in which there is only one round of DNA replication. This is in supposedly diploid sporophytes in which many of the cells have 4C to 16C DNA (Garbary and Clarke 2002). While kelps and fucoids are distantly related within brown algae (Bittner et al. 2008), these examples of the uncoupling of DNA replication from mitosis, and mitosis from cytokinesis show the scope of variation in the normal cell cycle among these organisms. However, in none of the photosynthetic systems (including the brown algae), is the production of anucleate cells in the absence of mitosis a dominant developmental pathway in nature. Rather, such anucleate cell systems have been formed experimentally via plasmolysis (e.g., Kroh and Knuiman 1988; Rutten and Derksen 1990; Galway et al. 1994; Pollock and Pickett-Heaps 2006), and various other techniques have been used to produce such cells in flowering plants (e.g., Lorz 1984), protozoa (Robinson et al. 1995) and yeasts (Kopecka et al. 1987). The resulting anucleate cells have become important experimental tools for studies as diverse as cell wall formation, the role of the cytoskeleton in cell morphogenesis, as well as cloning (e.g., Fulka et al. 2004). Thus, the naturally forming anucleate epidermal cells of Ascophyllum may represent a useful new tool for such purposes.
With respect to mitosis of Ascophyllum epidermal cells, we needed to determine if there was a diurnal pattern involved in this process. If such was the case, then this would require that observations of nuclear position be carried out at the time of maximal nuclear division. Kapraun and Boone (1987) described a distinct pattern of mitosis associated with the day-night cycle in several species of Scytosiphonaceae. In three species, a maximum of 8% of cells showed some stage of mitosis, and this occurred two hours after the onset of darkness. Our observations on Ascophyllum were unable to replicate this diurnal pattern, mitotic index was never greater than 5%, and there was no time of day when the mitotic index was significantly higher or lower. Furthermore, the low number of nuclei undergoing mitosis and absence of clustering of dividing meristoderm nuclei shows that such divisions cannot be associated with the formation of the epidermis that will be shed, since these cells are shed in a synchronous manner over large areas of the thallus (e.g., Filion-Myklebust and Norton 1981). Thus, the process is evidently synchronized either at the level of individual cell response to unknown internal or external signals, or coordinated through a process of cell-to-cell signaling within the meristoderm. Since brown algae produce virtually all of the known plant phytohormones (Tarakhovskaya et al. 2007), hormonal regulation of the process is a distinct possibility.
The dissociation of mitosis and cytokinesis may occur in other uninucleate algae. In Rhodophyta, spore germination of Gelidiales results in an anucleate cell following the migration of all of the cytoplasm into a germ tube (Guiry 1990). In the green alga, Blidingia minima, Garbary and Tam (1989) described a system in which an apical cell in a prostrate filament routinely elongated and formed a trailing cell devoid of nucleus or chloroplasts. Thus, while other algal groups have phenomena analogous to that described here for Ascophyllum, in that cytokinesis may be proceeding in the absence of mitosis, none are involved with the formation of a cell layer that is shed from the thallus. Toth (1976) described spore germination in Halosiphon tomentosa (as Chorda). He showed that what appeared to be empty spore germination was associated with an apparently rapid mitosis that left behind a small nucleus in the basal cell of the germ tube. Such rapid mitosis and subsequent migration of the apical daughter nucleus to the cell base might explain the absence of nuclei in the upper portions of cells. However, our diurnal study (every 4 h) does not support such a possibility. Furthermore, the possibility that epidermal cells are initially nucleated, and that these nuclei undergo degradation, can be discounted based on our 4 h sampling interval, and the reported minimum time course of 6 h for PCD in plant cells (review in Reape et al. 2008). Thus, the absence of any nuclei in the upper portions of cells out of the many tens of thousands that were observed, and the complete absence of synchrony in mitosis in the meristoderm is inconsistent with the formation of an epidermal layer with nucleated cells at any stage.
While the process of forming cells in the absence of mitosis might be considered highly unusual, the shedding of cells from the exterior of plants and algae is not. An analogous process to the epidermal shedding in Ascophyllum is the regular shedding of cells or blocks of cells from the margins of root caps (Hawes et al. 1998; Hamamoto et al. 2006; Driouich et al. 2007). This release of whole cells into the rhizosphere allows the plant to modify the surrounding environment and regulate potential pathogens and mutualistic symbionts. Key differences between these systems are the fact that the root cap cells are alive when they are shed and they remain alive for some period while they carry out their regulatory functions (Hawes et al. 1998; Hamamoto et al. 2006). In Ascophyllum, the shed cells would be removed by tides and waves.
Another analogous system to Ascophyllum is in the peanut, Arachis hypogaea. In this species, root epidermal cells can be shed as a layer following wall degradation by an endogenous cellulase (Uheda et al. 1997). These root epidermal cells are nucleated, but they senesce soon after release. During secondary thickening of Arabidopsis roots not only the epidermis, but also the adjacent cortex are shed from the plant as new growth occurs (Dolan et al. 1993). Again, these cells are initially nucleated, but their status when shed (i.e., living or dead) has not been further characterized. However, the process most similar to epidermal shedding in Ascophyllum occurs in red algae. In some calcified and noncalcified, crustose red algae there is a process whereby one or more layers of cells are regularly shed from the upper surface of the thallus. This process is considered to be an adaptation for the removal of epiphytes (e.g., Keats et al. 1997). The ontogeny of these cells is such that a process of programmed cell death follows cytokinesis, whereby the nucleus and other organelles progressively break down prior to shedding (Pueschel 1988; Pueschel and Miller 1996; Pueschel et al. 1996).
The basic strategy of Ascophyllum and other epidermal shedding fucoids is to produce an exfoliant layer that will remove fouling organisms from the algal surface when it is shed. Thus the absence of mitosis during the formation of this layer is adaptive. It is counterproductive for these algae to invest in both the materials and energetics of nuclei to package into the exfoliant layer, if this is only going to be shed. Thus we predict that the shed ‘skin’ has minimal cytoplasm, organelles and nutrient content because all of this material is going to be lost following exfoliation. This prediction is supported by preliminary observations that chloroplasts are excluded from the anucleate cells of the shed epidermal layer. Ultrastructure of meristoderm cells showed that the upper portions of these cells were highly vacuolated with small mitochondria aligned along the upper wall as the only conspicuous organelles (Xu et al. 2008). The only materials specifically concentrated in these cells are the physodes, which also function as antifouling agents (Schoenwaelder 2008), so their presence in the meristoderm and anucleate epidermal cells also appears to be adaptive for the anti-fouling function of the shedding process.
No discussion of Ascophyllum biology is complete without reference to its mutualistic fungal symbiont, Mycophycias ascophylli and its involvement, if any, with the process of epidermal shedding. All Ascophyllum fronds in nature have the fungus (Kohlmeyer and Kohlmeyer 1972; Garbary and Gautam 1989; Garbary and Deckert 2001). Experimental evidence that this was a mutualistic symbiosis was produced by Garbary and MacDonald (1995) and Garbary and London (1995) who showed that Ascophyllum embryos grew faster and had a different morphology when colonized by the fungus, and that the embryos were more tolerant of desiccation following colonization. More recently, Deckert and Garbary (2005b) and Garbary et al. (2005) showed that hyphae of Mycophycias had a highly organized arrangement in Ascophyllum, and that complex rings of hyphae formed around many cells in the host, in particular at the base of meristoderm cells and the outer cortex. While the fungus forms a systemic network throughout the host thallus, it is intriguing that it is never associated with the shed epidermal cells, and neither light nor electron microscopical studies revealed any indication of parasitism (Deckert and Garbary 2005a; Xu et al. 2008).
In conclusion, this study raises important questions for future research. For example, what normally determines the plane of cell division in brown algae, and does epidermal cell formation represent a variation on the normal process? Nagasato and Motomura (2002) argue that centrosomes determine the division plane, because they observe that the new wall forms between centrosomes, regardless of nuclear position, in their experimental system. In their review of cytokinesis, these authors conclude that "there is no spatial and temporal relationship between the mitotic spindle and cytokinesis in brown algae." The formation of the anucleate epidermis in Ascophyllum would be a prime example of this lack of association. Also, Hatzold and Conradt (2008) found that "asymmetric cell division and apoptosis can be functionally linked" in Caenorhabditis elegans. Thus, is the asymmetry significant in Ascophyllum, other than as representing a "minimum disposable volume"? The ability to address such questions in the absence of the complicating factor of mitosis suggests that epidermal development in Ascophyllum may be a useful model for future studies of cell division.