Reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical and hydrogen peroxide are physiological metabolites formed during aerobic life as a result of the metabolism of oxygen. High ROS levels induce oxidative stress, which can result in a variety of pathological conditions, including cardiovascular disease, cancer, and aging (Cox and Cohen 1996; Ames 1998).
As a natural antioxidant source, plants including algae have an ability to absorb the solar radiation for generating high levels of oxygen as secondary metabolites of photosynthesis. Oxygen is easily activated by ultraviolet (UV) radiation and heat from the sunlight to produce toxic ROS. Therefore, plants produce diverse antioxidative compounds to neutralize these ROS for its survival (Lu and Foo 1995). Although many studies regarding antioxidant effects from macroalgae are available, less attention has been paid for microalgae because of difficulties in the isolation and cultivation. Currently, microalgae are being paid more attention as nutraceutical and health food in the market. In the studies about biological composition influencing the nutritional value of microalgae, researchers have studied the levels of proteins that could be used as health food or animal feed (Brown and Jeffrey 1992; Fuentes
Synthetic antioxidant supplements such as butylated hydroxyanisol (BHA), butylated hydroxytolouene (BHT), α-tocopherol and propyl gallate (PG) have been used in order to reduce oxidative damages in human body (Sherwin 1990; Gulcin
Recently many researchers are interested in finding any natural antioxidants having safety and effectiveness, which can be substituted for current and commercial synthetic antioxidants, BHA and BHT. Microalgae have become good candidates for sources of natural antioxidants, as revealed by a number of recent studies (Hirata
1,1-Diphenyl-2-picrylhydrazyl (DPPH), thiobarbituric acid (TBA), trichloroacetic acid (TCA), sodium nitroprusside, sulphanilic acid, naphthylethylenediamine dihydrochloride, xanthine, xanthine oxidase from butter milk, nitro blue tetrazolium salt (NBT), butylated hydroxytoluene (BHT), α-tocopherol, 3-(2-Pyridyl)-5,6-di (p-sulfophenyl)-1, 2, 4-triazine disodium salt (ferrozine), potassium ferricyanide (K3Fe(CN)6), Folin-Ciocalteu reagent and linoleic acid were purchased from Sigma Co. (St Louis, USA). 2, 2-Azino-bis (3-ethylbenz-thiazolin)-6- sulfonic acid (ABTS), peroxidase and 2-deoxyribose were purchased from Fluka Chemie (Buchs, Switzerland). Digestive enzymes of food grades such as Viscozyme, Celluclast, AMG, Termamyl, Ultraflo, Protamex, Kojizyme, Neutrase, Flavourzyme and Alcalase were purchased form Novo Co. (Novozyme Nordisk, Bagsvaerd Denmark). All the other chemicals used were of analytical grades.
>
Sample collection and isolation
Fresh water microalgae sample were directly collected into one liter plastic bottles from the different places of the Mu-Soo Cheon in Jeju Island, Korea. Environmental factors such as water temperature and pH were measured at the sampling spot. Thereafter, recommended applying concentration of F/2 (Aquacenter Ltd. USA) media was added to the natural samples and the bottles were immediately transferred into the plant growth chamber (Vision, VS-3D, Korea) with the temperature of 20, 25 and 30°C and then it was kept for three days. The incubated samples were taken out and the pH was again measured and 1 mL sample was transferred to the S-R chamber for the observation of abundance and community changes of phytoplankton under an inverted microscope. Finally the single cell of
The mass culture of microalgae was done with F/2 nutrients media, 10% soil extract and distilled water. Culture condition was maintained with the temperature, pH, light intensity and L:D cycle of 25°C, 8.0, 180 μE m?1 S?1 and 12:12 for
>
Preparation of enzymatic digests
Freeze-dried fresh water microalgae sample were ground into a fine powder and one gram was mixed with 100 mL of distilled water. The optimum pH of the each reaction mixtures were adjusted with 1 M HCl/NaOH. Optimum pH and temperature conditions for the respective enzymes were similar as described by Heo
Proximate chemical composition of freeze-dried fresh water microalgae sample were determined according to the AOAC methods (1995). Crude lipid content was determined by Soxhlet method and crude protein content was determined by Kjeldhal method. Ash content was determined by calcinations in furnace at 550°C and the moisture content was determined keeping in a dry oven at 105°C for 24 h. In enzymatic digests, the crude protein content was determined by the Lowry method and the polysaccharide content was determined by phenol-sulfuric method.
>
DPPH free radical scavenging assay
The DPPH free radical scavenging activity of digests was measured by DPPH using the modified method of Brand-Williams
>
Hydrogen peroxide scavenging assay
The ability of the fresh water microalgae to scavenge H2O2 was determined according to the method of Muller (1995). Sample (80 μL) and 20 μL of 10 mM hydrogen peroxide were mixed with 100 μL of phosphate buffer (0.1 M, pH 5.0) in a 96-microwell plate and incubated at 37°C or 5 min. Thereafter, 30 μL of freshly prepared 1.25 mM ABTS and 30 μL of peroxidase (1 U mL?1) were mixed and incubated at 37°C for 10 min and the absorbance was measured at 405 nm.
>
Superoxide anion scavenging assay
Measurement of superoxide anion scavenging activity of microalgae digests was based on the method of Nagai
>
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was determined as described by Chung
>
Nitric oxide radical scavenging assay
Nitric oxide radical scavenging effect was determined according to the method reported by Garrat (1964). Two milliliter of 10 mM sodium nitroprusside in 0.5 mL of phosphate buffer saline (pH 7.4) was mixed with 0.5 mL of sample and incubated at 25°C for 150 min. From the incubated mixture 0.5 mL was taken out and added into 1.0 mL sulphanilic acid reagent (0.33% in 20% glacial acetic acid) then incubated at room temperature for 5 min. Finally, 1.0 mL naphthylethylenediamine dihydrochloride (0.1% w/v) was mixed and incubated at room temperature for 30 min and absorbance was measured at 540 nm.
>
Ferrous ion chelating effect
The chelating of ferrous ions by the digests was estimated by the method of Decker and Welch (1990). Sample (5 mL) was added to a solution of 0.1 mL of 2 mM FeCl2. The reaction was started by the addition of 0.2 mL of 5 mM ferrozine solution and reaction mixture was incubated for 10 min at a room temperature in a shaking incubator. After incubation, the absorbance of reaction mixture was measured at 562 nm.
>
Determination of lipid peroxidation inhibitory effect with the ferric thiocyanate (FTC) method
The lipid peroxidation inhibitory effect of fresh water microalgae was determined according to the ferric FTC method (Kikuzaki and Nakatani, 1993). Two milliliter of Sample (100 mg L?1) was mixed with 2 mL of 2.51% linoleic acid in ethanol, 4 mL of 0.05 M of phosphate buffer (pH 7) and 2 mL of distilled water and kept at 40°C in the dark. A total of 0.1 mL of the above mixture was added to 9.7 mL of 75% ethanol and 0.1 mL of 30% ammonium thiocyanate and after 5 min, 0.1 mL of 0.02 M ferrous chloride in 3.5% HCl was mixed. The absorbance was measured every 24 h for 7 days.
Total phenolic compounds in the digests were determined according to the method of Chandler and Dodds (1993) using gallic acid as a standard phenolic compound. Sample (1 mL) was mixed with 1 mL of 95% ethanol, 5 mL of distilled water and 0.5 mL of 50% Folin- Ciocalteu reagent. The mixture was allowed to react for 5 min and 1 mL of 5% Na2CO3 was added. After mixing thoroughly, the mixture was placed in the dark for 1 h then absorbance was measured at 725 nm.
Statistical analyses were conducted with the SPSS 11.5 version software package on the triplicate (n = 3) test data. The mean values of each treatment were compared using one-way analysis of variance (ANOVA) followed by Turkey’s test. A p-value of less than 0.05 was considered as significant.
The temperature and pH varied from 25 to 30°C and from 7.6 to 8.2, respectively in the natural condition. Incubated samples showed higher abundance of
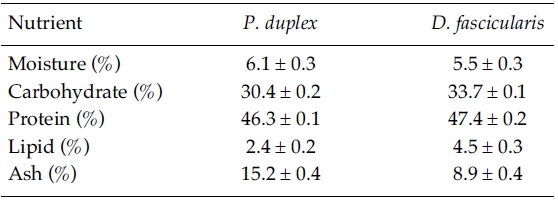
Proximate composition of freeze-dried
[Table 1.] Proximate composition of P. duplex and D. fascicularis
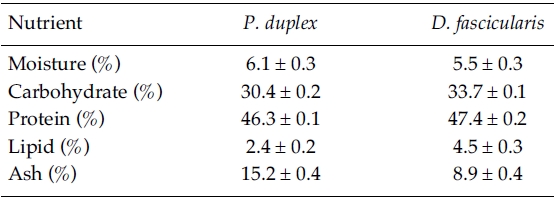
Proximate composition of P. duplex and D. fascicularis
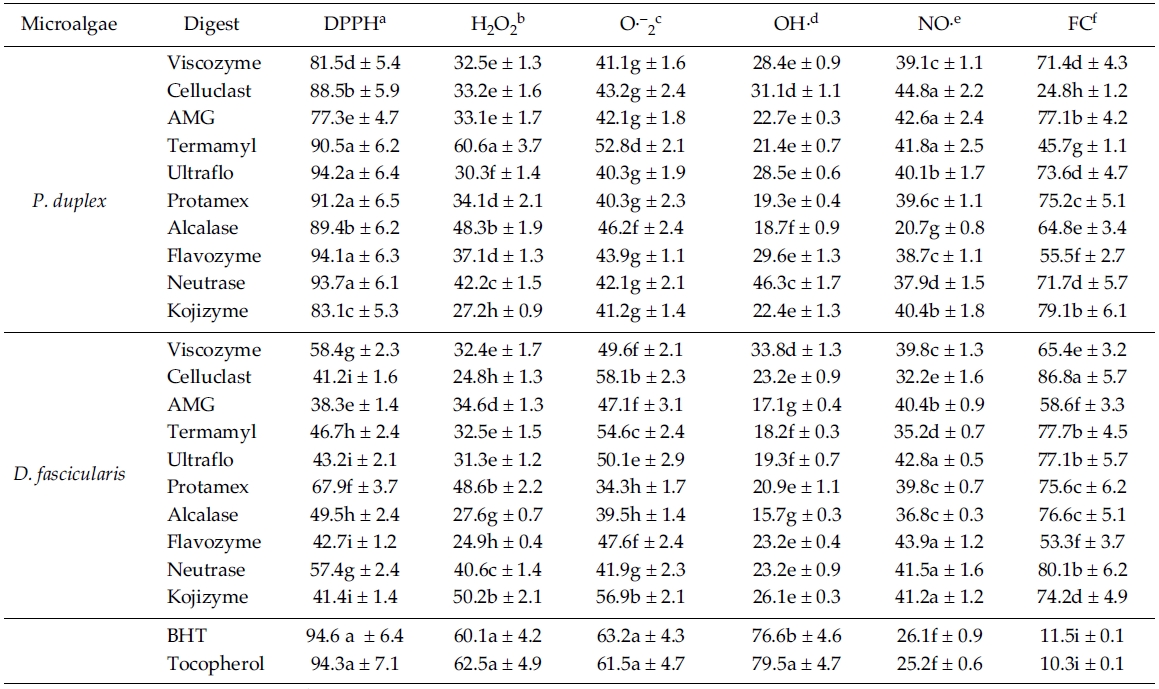
The percentage scavenging activity of enzymatic digests against DPPH are shown in Table 3. All enzymatic digests from
The H2O2 scavenging activities on enzymatic digests are shown in Table 3. Among the recorded results, Termamyl digest (60.6%) from
According to the Table 3, among all the enzymatic digests from
As shown in Table 3, enzymatic digests by Neutrase (46.3%), Celluclast (31.1%) from
Nitric oxide scavenging effect of enzymatic digests is shown in Table 3, almost all the enzymatic digests have shown significantly higher activities compared to the commercial antioxidants (
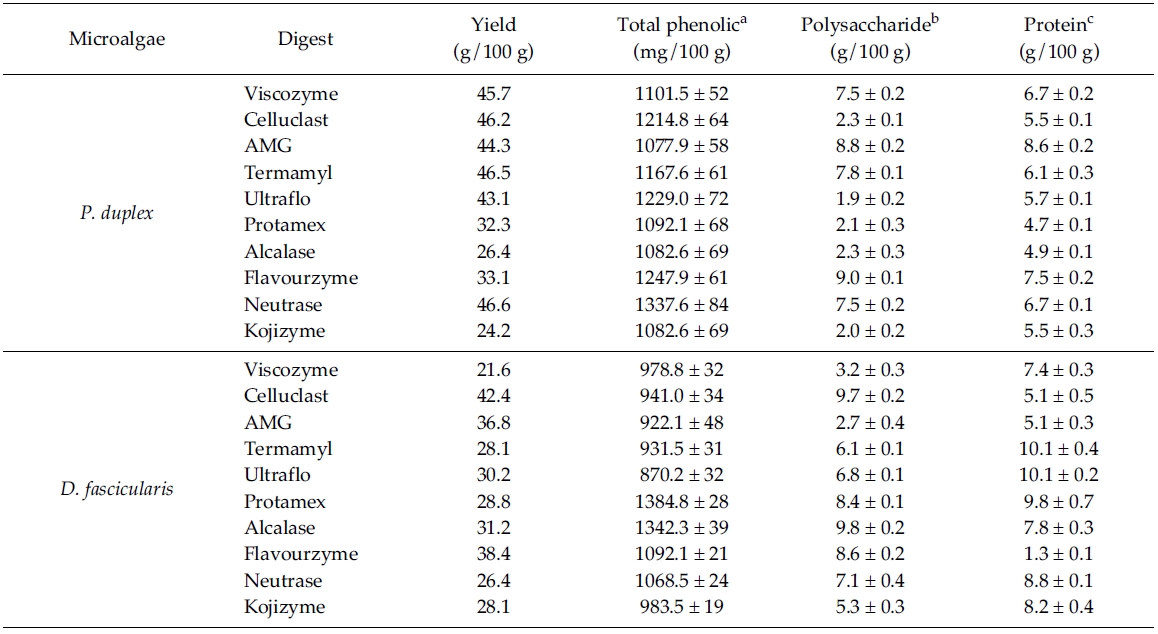
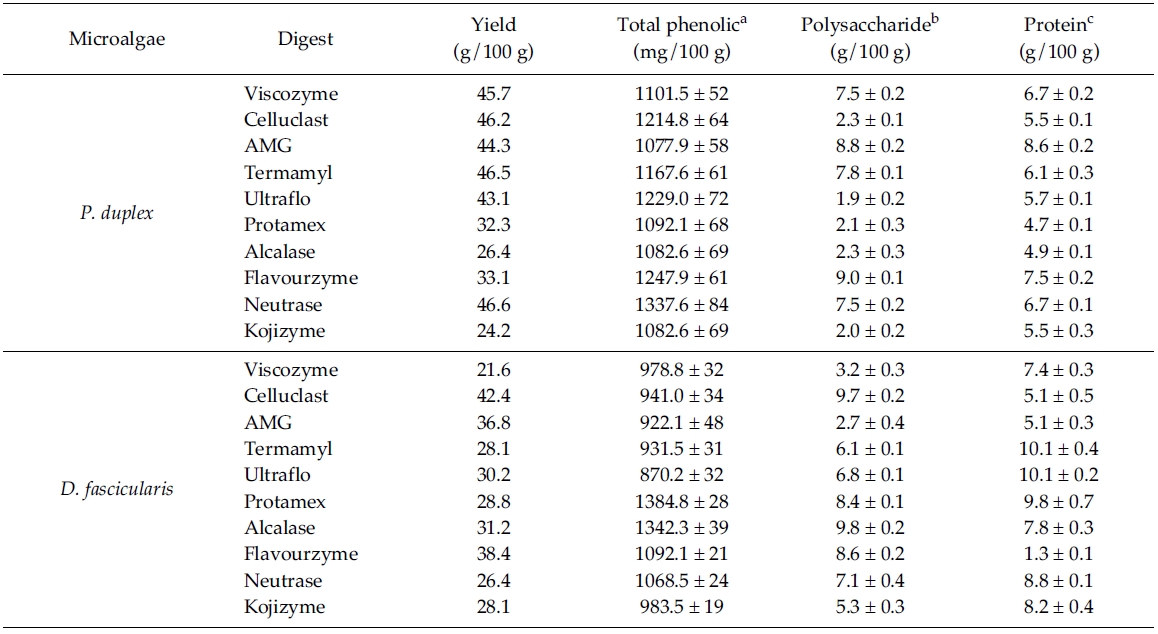
Total phenolic, polysaccharide and protein content of different enzymatic digests from P. duplex and D. fascicularis
[Table 3.] Antioxidant activities of enzymatic digests from P. duplex and D. fascicularis
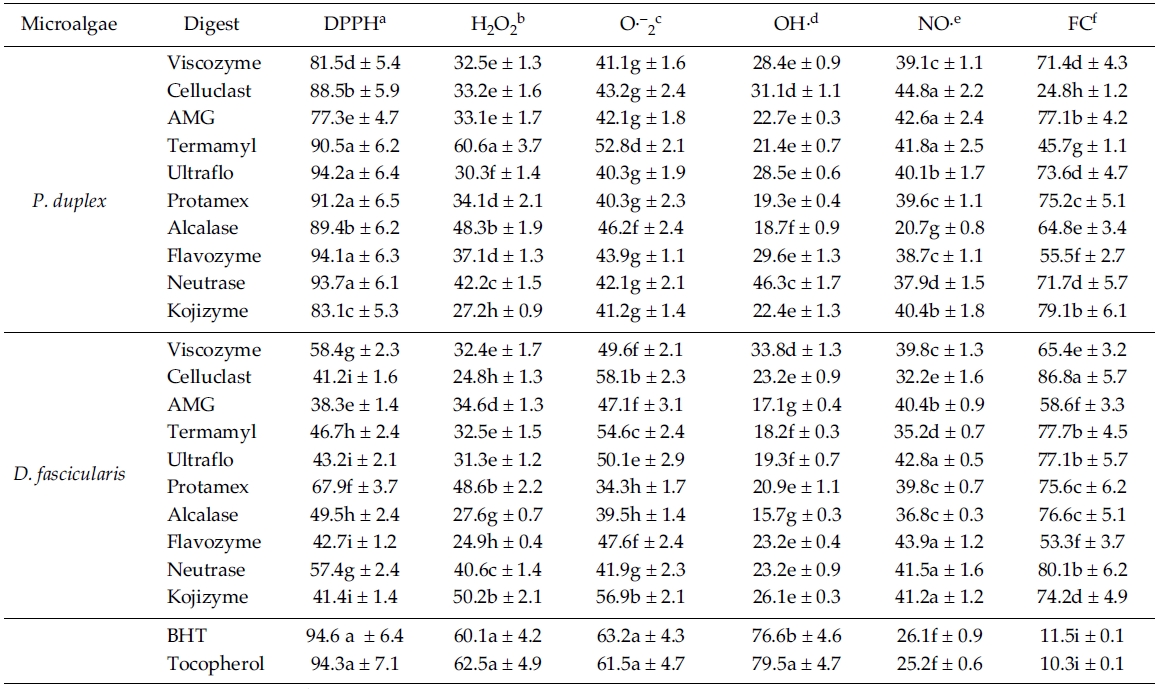
Antioxidant activities of enzymatic digests from P. duplex and D. fascicularis
According to the Table 3, all the enzymatic digests have exhibited strong ferrous ion chelating effects even significantly (
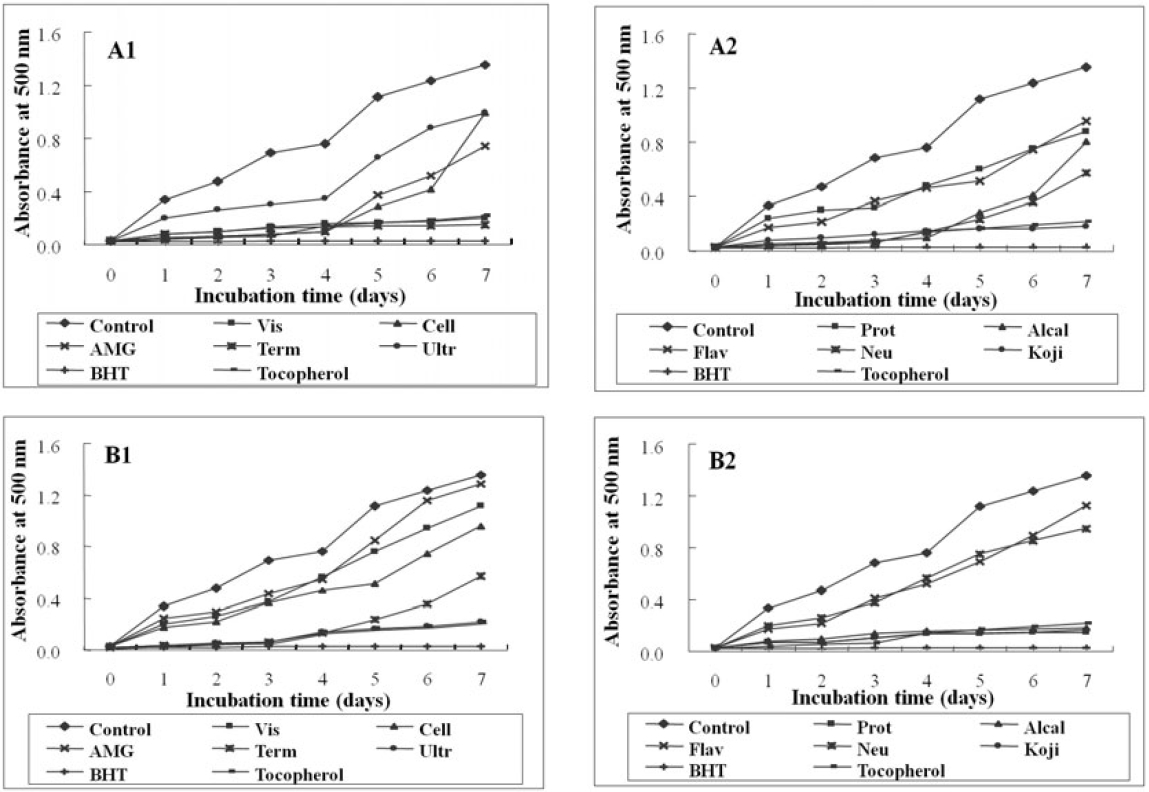
As shown in Fig. 1, the absorbance of linoleic acid emulsion without the addition of any digest was increased. Enzymatic digests from
We presently focused on natural water-soluble antioxidants from two species of the Jeju freshwater microalgae, which was prepared by enzymatic digestion using five carbohydrases (Viscozyme, Celluclast, AMG, Termamyl and Ultraflo) and five proteases (Protamex, Kojizyme, Neutrase, Flavourzyme and Alcalase) and potential antioxidant activities of the resultant enzymatic digests were evaluated using different reactive oxygen species (ROS) scavenging assays, ferrous ion chelating effect and lipid peroxidation inhibitory activity.
DPPH is a free radical generating compound and has been widely used to evaluate the free radical scavenging ability of various antioxidative compounds. DPPH free radical scavenging activity of all enzymatic digests from
Although H2O2 itself is not very reactive, it may convert into more reactive species such as singlet oxygen and hydroxyl radicals. Thus, removing H2O2 is very important for the protection of living systems. Addition of H2O2 to cells in culture can lead to transition metal ion-dependent HO. mediated oxidative DNA damage (Halliweill 1991). According to the H2O2 scavenging activity results, all the enzymatic digests exhibited activities indicating the efficiency of scavenging of H2O2 by both microalgae.
Superoxide anion and hydroxyl radicals are the two most effective representative free radicals. In cellular oxidation reactions, superoxide anion radical is normally formed first and its effects can be magnified because it produces other kinds of cell damaging free radicals and oxidizing agents (Liu and Ng 2000). In this study, the superoxide anion scavenging activities of carbohydrase digests of
Hydroxyl radical scavenging activity of the enzymatic digests from fresh water microalgae was determined as the percentage of inhibition of hydroxyl radicals generated in the Fenton reaction mixture. According to the hydroxyl radical scavenging activity results, all the enzymatic digests of
Nitric oxide is a gaseous free radical, which has important roles in physiological and pathological conditions. Enzymatic digests exhibited considerable effects which could be attributed to their hydrophilic properties. The reactivities of the NO· and O·? 2 were found to be relatively low, but their metabolite ONOO- (peroxynitrite) is extremely reactive and directly induce toxic reactions, including SH-group oxidation, protein tyrosine nitration, lipid peroxidation and DNA modifications (Radi
Ferrous is known as the most important lipid oxidizing prooxidant among the transition metals due to its high reactivity. Ferrozine can make red color complexes with ferrous ions. In presence of chelating agents, complex formation is interrupted and as a result, the red color of the complex is decreased. All the enzymatic digests from fresh water microalgae indicating significant chelating ability, which further support the idea that the hydrophilic components are accountable for ion chelating. Ferrous ions accelerate peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals (Halliweill 1991; Fridovich 1995). In this study, different digests of fresh water microalgae demonstrated a noticeable capacity for iron binding, suggesting their ability as peroxidation protector, which relates to their ferrous binding capacity (Gulcin
To evaluate the lipid peroxidation inhibition effect of microalgae digests, their lipid peroxidation were compared with the commercial antioxidants using FTC method by determining the amount of peroxide formed in emulsion during incubation period. High absorbance is an indication associated with high concentration of formed peroxides. In this experiment, the enzymatic digests from
The cell wall of