The etching characteristics of zinc oxide (ZnO) were investigated, including the etch rate and the selectivity of ZnO in a Cl2/BCl3/Ar Plasma plasma. It was found that the ZnO etch rate, the RF power, and the gas pressure showed non-monotonic behaviors with an increasing Cl2 fraction in the Cl2/BCl3/Ar Plasma plasma, a gas mixture of Cl2(3 sccm)/BCl3(16 sccm)/Ar (4 sccm) resulted in a maximum ZnO etch rate of 53 nm/min and a maximum etch selectivity of 0.89 for ZnO/SiO2. We used atomic force microscopy to determine the roughness of the surface. Based on these data, the ion-assisted chemical reaction was proposed as the main etch mechanism for the plasmas. Due to the relatively low volatility of the by-products formed during etching with Cl2/BCl3/Ar Plasma plasma, ion bombardment and physical sputtering were required to obtain the high ZnO etch rate. The chemical states of the etched surfaces were investigated using X-ray photoelectron spectroscopy (XPS). This data suggested that the ZnO etch mechanism was due to ion enhanced chemical etching.
Recently, a ZnO thin film was produced, greatly enhancing the possible devices which can be manufactured. In particular, the ZnO thin film has been used by many research groups to fabricate optically transparent thin film transistors. Important aspects of ZnO thin film are a band-gap energy of 3.37 eV and a binding energy of ~ 60 meV. Also, the ZnO thin film exhibits increased radiation resistance, which is important for devices used in aerospace and nuclear applications. Finally, the ZnO thin film can be grown on inexpensive substrates, such as glass, at relatively low temperatures [1-4].
Until now, there has been little research devoted to the etching characteristics of ZnO thin films using both fluorine- and chlorine-based plasma chemistries. Research has been conducted that shows the dependences of the ZnO etch rate on the operating conditions using Cl2/Ar, BCl3/Ar, CH4/H2, C2H6/H2, IBr/ Ar, BI3/Ar and CH4/H2/Ar plasmas; however, the etch mechanisms, the relationships between the process parameters, and the chemistry involved are not discussed [5-8]. However, there are only a few studies related to ZnO dry etching using the high density plasma sources favored by modern microelectronic technology. As a result, the influence of the input process parameters on the ZnO etch rate have not been explored in detail, and the ZnO etch mechanism remains unclear.
In this work, we investigated the etching characteristics and mechanisms of ZnO thin films using Cl2/BCl3/Ar mixtures in an inductively coupled plasma (ICP) system. The chemical reaction on the surface of the etched ZnO thin film was investigated using X-ray photoelectron spectroscopy (XPS).
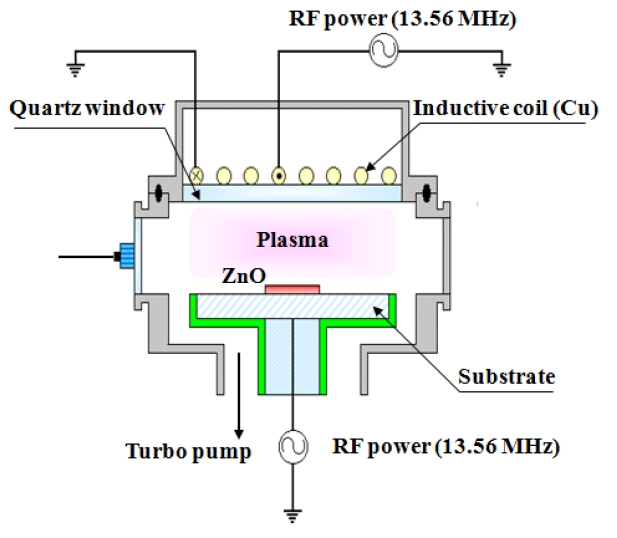
The ZnO thin films were prepared on Si (100) substrates using a traveling wave-type ALD reactor (Evertek, Plus 100, Korea). The vaporization temperature of the ZnO films was 15℃, and the final thickness of the ZnO films was about 200 nm. Etching experiments were performed in a planar ICP system, as shown schematically in Fig. 1. The reactor chamber was made from aluminum, and the inner walls were anodized. A 3.5 turn spiral induction coil was located above a 24-mm-thick horizontal quartz window that separated the coil from the process chamber. The height of the working zone, i.e., the distance between the quartz window and the bottom electrode, was 14 cm. The bottom electrode (used as the substrate holder) was connected to an asymmetric RF 13.56 MHz generator. The ZnO thin films were etched in Cl2/BCl3/Ar Plasma plasma, and the process variables were the gas mixing ratio, the process pressure (1-3 Pa), and the radio frequency (RF) power (600-800 W). In all the experiments, the total gas flow rate, the RF power, the DC-bias voltage, and the substrate temperature were fixed at 20 sccm, 700 W, -150 V, and 35℃, respectively. The etch rates were measured using an
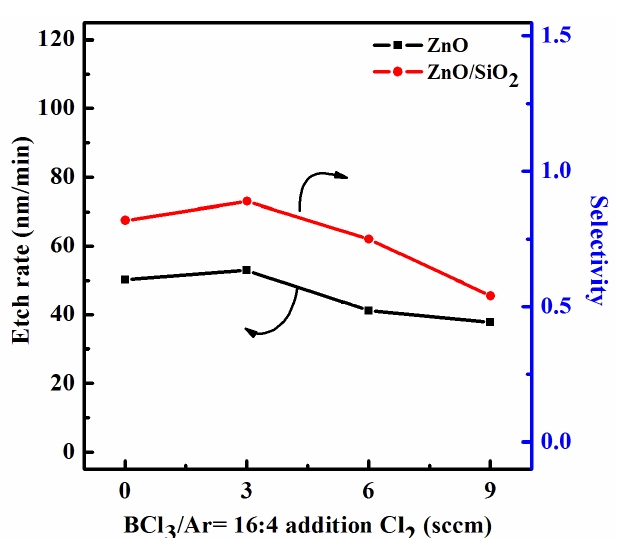
Figure 2 shows the etch rate of the ZnO thin film and its selectivity to SiO2 as a function of the Cl2/BCl3/Ar Plasma mixing ratio at a process pressure of 2 Pa, an RF power of 700 W, and a DC-bias voltage of -150 V. It can be seen that, as the Cl2 (3 sccm) content in the BCl3/Ar plasma increased, the etch rate of the ZnO thin films also increased, reaching a maximum of 53 nm/min at a Cl2 (3 sccm)/BCl3 (16 sccm)/Ar (4 sccm) gas mixing ratio. The etch rates of the ZnO in Cl2 (0 sccm)/BCl3 (16 sccm)/Ar (4 sccm) and Cl2 (9 sccm)/BCl3 (16 sccm)/Ar (4 sccm) were found to be 40 nm/min and 37.7 nm/min, respectively. These findings support the assumption that, for a given range of experimental conditions, increasing the chemical reaction gas concentration decreases the etch rate. The selectivity of the ZnO to the SiO2 increased from 0.82 to 0.89 until a Cl2 (3 sccm)/ BCl3 (16 sccm) /Ar (4 sccm) plasma occurred. The result was a lowering of the rate of the chemical reaction in the BCl3-rich plasma due to the deposition of solid inorganic compounds that resulted from a complete dissociation of the BCl3 molecules. It was shown that the variation in the gas mixing ratio caused monotonic changes in the electron density, the electron temperature, the total ion density, and the Cl atom density. Accordingly, we can assume the same monotonic behaviors in the fluxes of these species, and the first of the proposed mechanism can be neglected. It is our opinion that the second and third mechanisms operate simultaneously.
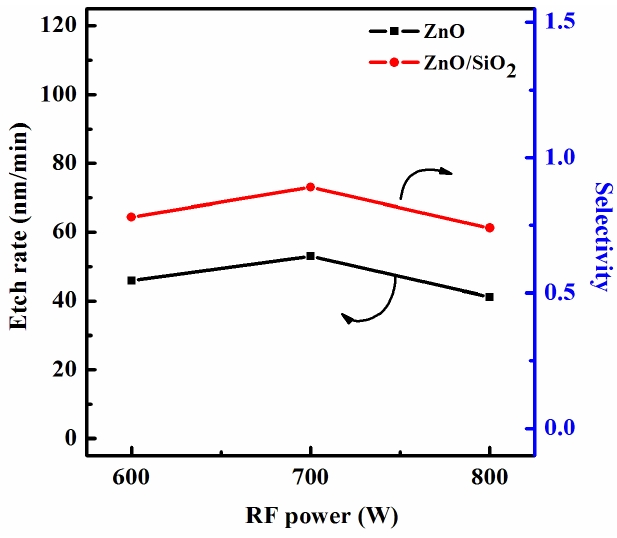
Figure 3 shows the etch rate of the ZnO thin films and the etch selectivity of the ZnO to SiO2 as a function of the RF power for the Cl2 (3 sccm)/BCl3 (16 sccm)/Ar (4 sccm) plasma. As the RF power increased, the ZnO etch rate increased from 35.9 nm/min at 600 W to 53 nm/min at 700 W, the selectivity of ZnO to SiO2 increased from 0.6 to 0.75. However, at 800 W, the ZnO etch rate and selectivity to SiO2 decreased rapidly down to 34 nm/min and 0.64, respectively. An increase in the RF power causes monotonic increases in both dissociation and the ionization rates, and thus in the densities and fluxes of the Cl atoms and positive ions. In our case, such a layer can result from the deposition of solid B that then bondeds with the surface oxygen to form BxOy, as well as from the B and Cl radicals incorporated in the polymer-like structure [9].
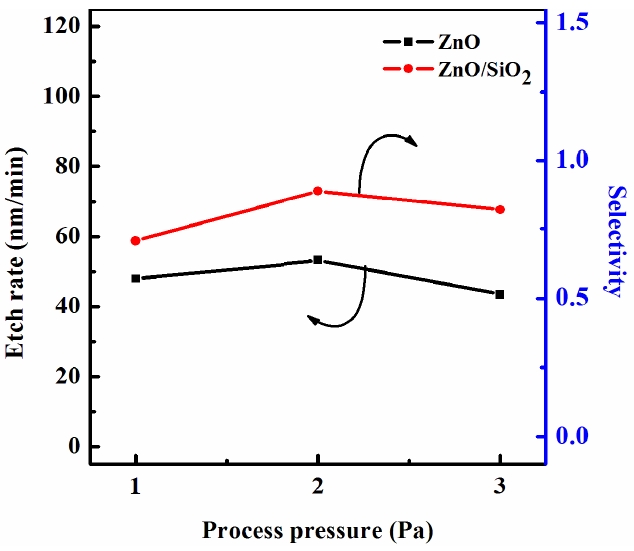
Figure 4 shows the ZnO thin film etch rate and selectivity to SiO2 as a function of the process pressure for the Cl2 (3 sccm)/ BCl3 (16 sccm)/Ar (4 sccm) gas mixing ratio. As the pressure increased from 1 Pa to 2 Pa, the ZnO etch rate increased from 43 nm/min to 53 nm/min, and it then showed a saturation trend as it decreased to 3 Pa. The selectivity of the ZnO to SiO2 increased from 0.61 to 0.89 and then decreased rapidly down to 0.82 at 3 Pa. In our opinion, the effect of the gas pressure is as follows. An increase in the gas pressure enhances the density of the neutral chemically active species but lowers the ion mean free path and ion energy. As a result, with an increasing gas pressure, there is a tendency toward acceleration in the chemical etch pathway but a worse condition for the ion stimulated desorption of the reaction products, which likely results in a decreased fraction of the free surface acceptable for chemical reaction. Similar to the effect of the gas mixing ratio, these two factors work in opposing directions, producing a non-monotonic behavior in the etch rate.
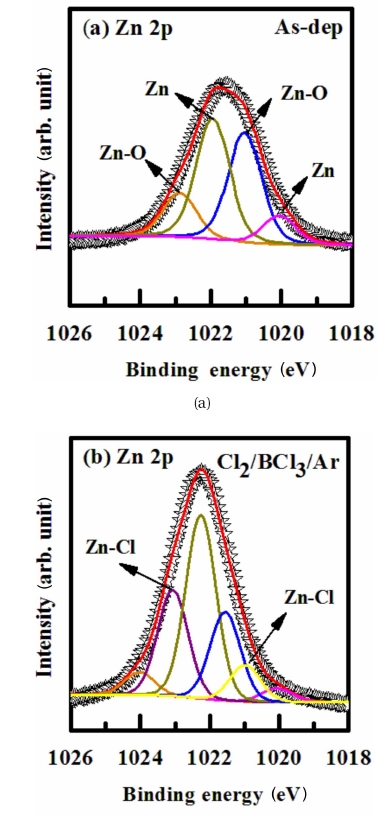
Figure 5 shows the XPS narrow scan spectra for Zn at a 90° angle. The spectra of the ZnO film surface were collected before and after etching in a Cl2/BCl3/Ar Plasma mixture. The peaks obtained from the as-deposited ZnO film were used as a reference. For the as-deposited film, the total Zn 2p peak can be decomposed into four peaks at Zn-O (1,021.04 eV, 1,022.89 eV) and at metallic Zn (1,020.09 eV, 1,021.94 eV). The Zn-O bond (1,021.04±0.5 eV, 1,022.89±1.15 eV) and metallic Zn (1,020.09±0.1 eV, 1,021.94±0.35 eV) XPS scan spectra of the ZnO surface were etched with Cl2/ BCl3/Ar plasma. For the films etched in the chlorine containing plasmas, new peaks at 1,020.99 eV and 1,023.14 eV appeared in the Zn 2p spectra. The high energy of this peak shifted with an increasing BCl3, Cl2 and Ar mixing ratio; thus, we could relate it to the signal from the Zn-Clx, Zn-BCly [10].
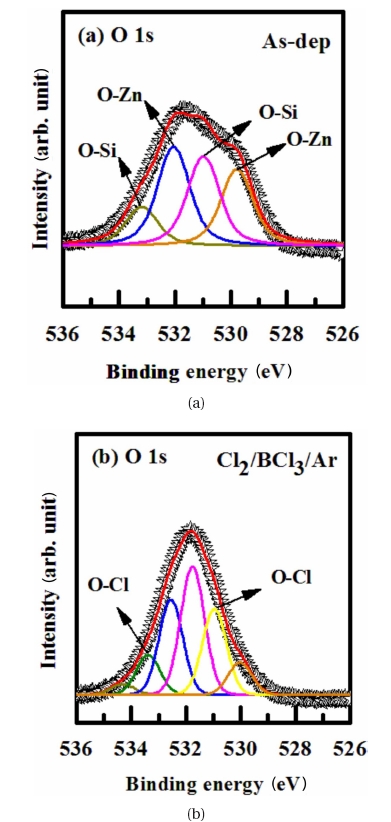
As shown in Fig. 6 for O 1s, after etching the ZnO thin film in Cl2/BCl3/Ar Plasma plasma, the peaks obtained from the as-deposited ZnO film were used as a reference. For the as-deposited film, the total O 1s peak can be decomposed into four peaks at O-Zn (529.8 eV, 532 eV) and O-Si (531 eV, 533 eV). However, in the ZnO thin film that was etched in Cl2/BCl3/Ar Plasma gas, the O 1s peak intensities at O-Zn (529.8±0.2eV, 532±0.6 eV) and O-Si (531±0.75 eV, 533±1.3 eV) increased due to the formation of B-O and Cl-O bonds. This peak intensity of O 1s in Cl2/BCl3/Ar Plasma is higher than that in the asdeposited film, which demonstrates a dramatic increase in the B-O bonds and Cl-O bonds, such as exist in B2O3, due to the dissociation of the O-Zn bonds by ion bombardment. For the films etched in the chlorine containing plasmas, new peaks at 531 eV and 533.4 eV appear in the O 1s spectra. There is also the possibility of O-Cl bond formation, even though that was not demonstrated here. The XPS results reveal that Zn and O are removed through chemical reactions with the B and Cl radicals and by the physical bombardment of Ar ions [11, 12].
In this work, we carried out experimental investigations of the ZnO etching mechanism in Cl2/BCl3/Ar Plasma plasma. We found that an increase of the Cl2 content in BCl3/Ar plasma leads to an increase in the ZnO etch rate, which reaches a maximum value of 53 nm/ min at a concentration of 3 sccm Cl2 because a large addition of Cl2 to the BCl3/Ar plasma increases the chemical reaction effect. Due to the relative volatility of the by-products formed during the Cl2/BCl3/Ar Plasma plasma etching, ion bombardment was required in addition to physical sputtering to obtain these high ZnO etch rates. The reason for this etch rate maximum is due to the concurrence of both physical and chemical pathways in the ionassisted physical reaction. Volatile oxygen compounds identified in the XPS results indicate that Zn 2p and O 1s form nonvolatile metal chlorides and volatile boron-oxy-chlorides. These results agree with the universal energy dependency of the ion enhanced chemical etching yields.