The interaction of ?Al(CH3)2 with ?OH on a fully OH?terminated Si (001) surface was studied using density functional theory. Two sites for Al(CH3)3 to react with the ?OH on the surface were identified. The ?Al(CH3)2 product energetically favored the dimer?row site rather than the inter?row site because the Al atom of ?Al(CH3)2 at the dimer?row site was attracted by the lone pair electrons of the O atom in the neighboring ?OH. The energy barrier for the transfer of the ?Al(CH3)2 between the two sites was 0.11 eV, and therefore, the ?Al(CH3)2 at the inter?row site can easily transfer to the dimer?row site at room temperature.
Functional one?dimensional metallic oxides have attracted much at gate oxides for metal oxide semiconductor field effect transistors (MOSFETs) have been fabricated using atomic layer deposition (ALD) because this technique allows the uniformity and thickness of the gate oxide film to be delicately controlled [1], [2]. Among the potential gate oxide materials, aluminum oxide (Al2O3) has superior physical and electronic properties including high band gap (∼9 eV), high dielectric constant (∼9), and high breakdown field (5?10 MV/cm) [3]?[5]. Various aluminum precursors including AlCl3, AlMe3, AlBr3, AlMe2Cl, AlEt3, Al(OEt)3, and Al(OiPr)3 have been employed with O2, O3, H2O, and H2O2 reactants for the growth of high quality Al2O3 thin films using the ALD process [6]?[14].
Recently, many studies on tri?methylaluminum (TMA, AlMe3) have been performed using density functional theory (DFT) [15]?[19]. Widjaja and Musgrave [15], Heyman and Musgrave [16] carried out ALD half reactions with TMA, AlCl3, and H2O. In addition, Lee et al. [17] were able to prepare a hydroxyl (OH)?terminated Si (100) 2 × 1 surface. Halls and Raghavachari [18] calculated the growth reaction of Al2O3 with TMA on a reconstructed H?terminated Si (100) surface. Recently, Ghosh and Choi [19] researched the initial growth mechanism of Al2O3 using TMA and H2O on a fully OH?terminated Si (100) 2 × 1 surface. They found that the fully OH?terminated Si surface allowed a ring?closing reaction, yielding a unique fivemembered ring structure, essentially saturating the surface bonding sites between the Si surface and the Al2O3 junction region. However, the main focus of that work was on the TMA reaction with ?OH along the path of the dimer?row site, and the effect of the lone pair electrons of the ?OH near the di?methylaluminum (DMA) group (?Al(CH3)2) was only briefly mentioned.
In this study, we present two reaction sites of TMA with the ?OH on a fully OH?terminated Si (001) surface, and investigate the effect of the lone pair of ?OH electrons on the stability of ?Al(CH3)2. In addition, the transfer energy barrier of ?Al(CH3)2 is calculated using the nudged elastic band (NEB) tool [20].
DFT calculations were performed using the Vienna ab?initio simulation package (VASP) code with the projector augmented wave (PAW) potentials, an all?electron method that combines the accuracy of augmented plane waves, the cost? effective pseudopotential implemented in VASP, and the generalized gradient approximation (GGA) [21]?[24].
The residual minimization method of direct inversion in the iterative subspace (RMM?DIIS) was used for calculating the ground state of electrons [25], [26]. More precisely, the Al35s2 and 3p1 states, O 2s2 and 2p4 states, C 2s2 and 2p2 states, H 1s1 state, and Si 3s2 and 3p2 states were treated as valence wave functions. The cutoff energy was 500 eV, and the Monk? horst pack k?points mesh was 2×2×1 for the slab structure.
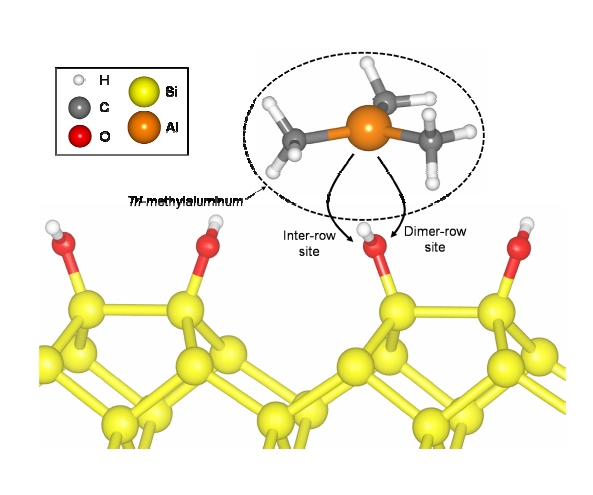
The most energetically favorable Si (001) 4×4 surface that was fully OH?terminated was first determined by calculation. H atoms of the ?OH groups that were attached to the Si dimers of the surface were aligned along the dimer?row direction in zigzags. There were two sites for the TMA transfer from the vacuum to the surface, the inter?row site and dimerrow site, when TMA approached and reacted with ?OH on the fully OH?terminated Si (001) surface, as shown in Fig. 1. The inter?row and the dimer?row sites indicate the sites of TMA with respect to ?OH attached to a Si atom of the Si dimer. The reaction between TMA and ?OH generated methane (CH4) as a by?product and the remaining TMA fragment, ?Al(CH3)2, was attached to the ?O radical. Si surfaces with ?Al(CH3)2 located at the two sites were calculated by DFT and the resulting electron densities of ?Al(CH3)2 groups were analyzed using the visualization for electronic and structural analysis (VESTA) tool [27]. In addition, the energy barrier was calculated when ?Al(CH3)2 transferred from the inter?row site to the dimer?row site using the NEB tool.
The chemical formula of the TMA reaction with ?OH is given by Si?OH + Al(CH3)3 → Si?O?Al(CH3)2 + CH4 [19]. Fig. 2
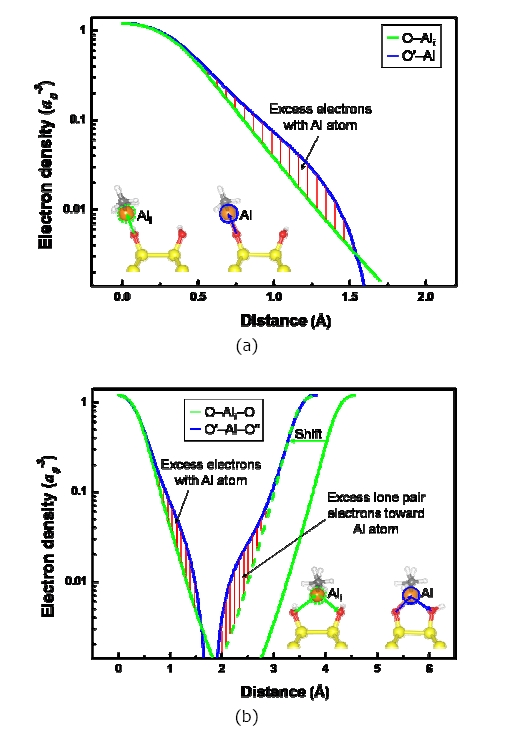
shows the charge density distribution and the transfer of atoms when ?Al(CH3)2 is located at the inter?row site or the dimer?row site of the fully OH?terminated Si (001) surface along the [110] (Figs. 2(a) and (c)) and [001] (Figs. 2(b) and (d)) directions. An isosurface level is the amount of electron charge per a0 3 (a0 = Bohr radius); for this case its value in Fig. 2 is 0.063. O′ and O′′ in Fig. 2 indicate the oxygen atom reacting with the Al atom of ?Al(CH3)2 and the oxygen atom of ?OH attached to the other Si atom of the same dimer, respectively. The O′ atom of ?O′H was bonded with the Al atom of ?Al(CH3)2 when TMA transferred to the inter?row site and reacted with ?O′H, as shown in Figs. 2(a) and (b). H atoms of the ?OH groups located near the two CH3 groups of ?Al(CH3)2 were repelled (indicated by the blue arrows for the H atoms). The O′?Si?Si angle and the distance between O′ and O′′
[Table 1.] The angles and distance between two atoms around ?Al(CH3)2.
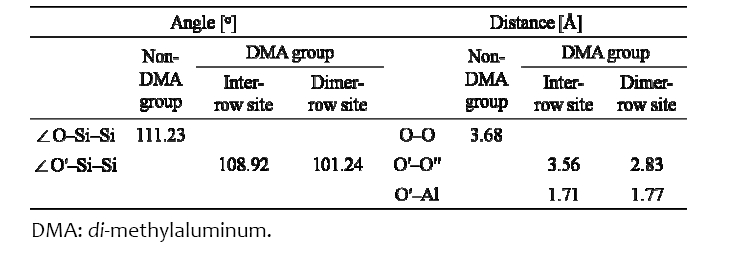
The angles and distance between two atoms around ?Al(CH3)2.
atoms were decreased by 2.08% and 3.26%, respectively, because the O′ atom transferred under the influence of the ?Al(CH3)2, as shown in Table 1. Si atoms were covalently bonded to Si atoms, whereas O atoms were mainly ionically bonded to Si atoms. The O′ atom was bonded with the Al atom of ?Al(CH3)2 by the p orbital electron of the O′ atom. When the ?Al(CH3)2 was located at the inter?row site, where there was enough space for TMA to react with the OH group, the energy was ?1.76 eV, which was 0.12 eV higher than the value of Halls and Raghavachari [18]. The difference may be due to the differently terminated Si (001) surface since they used a ½ OH? and ½ H?terminated Si (001) surface. When ?Al(CH3)2 was located at the dimer?row site between the O′ atom and ?O′′H, as shown in Figs. 2(c) and (d), the H atom of ?O′′H located near the ?Al(CH3)2 transferred farther away from ?Al(CH3)2 than in the case of the inter?row site, while the O atoms (O′ and O′′) transferred closer to each other (indicated by red arrows near the O atoms). In addition, the O′′?H bond length was decreased to 0.76 Å from 0.97 Å (i.e., by 21.65% as the O?H bond length located in the other sites was 0.97 Å), and the O′′?H bond rotated clockwise by approximately 55°. This indicates that the lone pair electrons of the O′′ atom transfer toward the Al atom of ?Al(CH3)2 to create a bond between the O′′ and Al atom. The H atom located on the other dimer?row site was transferred (indicated by the orange arrow) because it was influenced by the H atom of ?O′′H due to repetition of the 4×4 size of the Si surface in this study. In this situation, the O′?Si?Si angle and the distance between O′ and O′ atoms were further decreased by 9.0% and 23.1%, respectively, relative to the case of the inter?row site because of the lone pair electrons of the O′′ atom. ?Al(CH3)2 at the dimer?row site was energetically more favorable than that at the inter?row site by 0.43 eV, as shown in Fig. 2. The O′?Al bond length was increased compared to that of the inter?row site, as shown in Table 1, indicating that the Al atom was attracted to the O′′ atom.
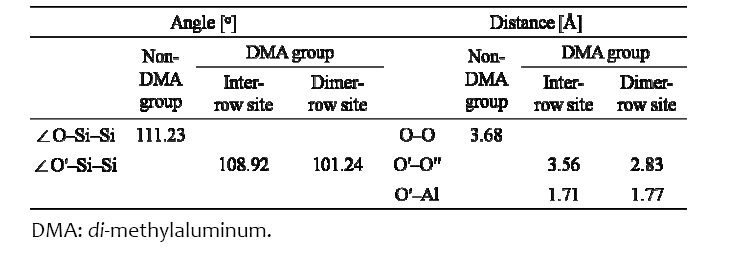
Figure 3 shows the line profiles of the electron densities of the O′?Al and O′′?Al, which are the numerical values calculated by the linear interpolation of the volumetric data. The imaginary Al atoms (Ali, a green dotted circle in the inserted picture) were produced for comparison. When ?Al(CH3)2 was at the inter?row site, the electron distributions around the non?bonded (green line, as shown in Fig. 3) and the bonded (blue line, as shown in Fig. 3) O atoms were different due to the Al atom, as shown in Fig. 3(a). We found that there was excess electron density at the O′?Al bond compared to that at the O?Ali bond because of the electron redistribution of the O′ atom to the O′?Al bond. When ?Al(CH3)2 was at the dimer?row site, the O′′ atom transferred toward the Al atom
to form a bond by using the lone pair electrons of the O′′ atom, as shown in Fig. 3(b). The green dotted line was the shifted line from the green line to be compared with the blue line directly because the distance of the O?Ali?O was longer than that of the O′?Al?O′′. In Fig. 3(b), we can clearly see that the lone pair electrons of the O′′ atom are located between the O′′ and the Al atoms as the excess electron density of the O′′?Al bond. The excess electrons of the O′′ atom were two times richer than the excess electrons of the O′ atom because those of the O′′ atom had the lone pair electrons. Some electrons of the H atom of the O′′?H also transferred to the O′′?Al bond, which was indicated by the reduction of the O′′?H bond length, as shown in Fig. 2(d).
Figure 4 shows the calculated energy barrier of the ?Al(CH3)2 transfer from the inter?row site to the dimer?row site, i.e., 0.11 eV. Since the energy barrier is quite small, the ?Al(CH3)2 can easily transfer from the inter?row site to the dimer?row site. Therefore, the ?Al(CH3)2 would primarily be located at the dimer?row site at room temperature.
We studied the interaction of ?Al(CH3)2 with ?OH on a fully OH?terminated Si (001) surface in order to determine the mechanism of the initial Al2O3 growth using DFT. There were two sites when TMA approached and reacted with ?OH on the fully OH?terminated Si (001) surface. ?Al(CH3)2 favored the dimer?row site rather than the inter?row site, because the lone pair electrons of the O atom of ?OH located next to the ?Al(CH3)2 on the same Si dimer attracted the Al atom of ? Al(CH3)2 and reduced the overall total energy. Based on the low energy barrier from the inter?row site to the dimer?row site, the ?Al(CH3)2 should mostly remain at the dimer?row site at room temperature.

![The charge density of ?Al(CH3)2 at (a) the inter?row site and (c) the dimer?row site along the [110] direction, and the planar view of ?Al(CH3)2 at (b) the inter?row site and (d) the dimer?row site along the [001] direction. The blue, orange, and red arrows represent the transfer directions of the H and O atoms.](http://oak.go.kr/repository/journal/10136/E1TEAO_2010_v11n1_11_f002.jpg)