Three biological aerated filters (BAFs) composed of a PVC pipe with a diameter of 75 mm were constructed and operated at a wastewater temperature at 13℃ The media used for each BAF were: 5-mm gravel; 5-mm lava rock; 12.5-mm diameter by 15-mm long plastic rings, all with a media depth of 1.7 m. The feedwater, which simulated the effluent of aerated lagoons, had influent soluble chemical oxygen demand (sCOD) and ammonia concentrations of approximately 50 and 25 mg/L, respectively. For a hydraulic retention time (HRT) of two hours without recirculation, ammonia percent removals were 98.5, 98.9, and 97.8%, for the gravel, lava rock, and plastic rings, respectively. By increasing the effluent recirculation from 100 to 200% for an HRT of one hour, respective ammonia removals improved from 90.1 to 96, 76.5 to 90, and 65.3 to 79.5% for gravel, lava rock, and plastic rings. Based on the ammonia and sCOD loadings for different HRTs, the estimated maximum ammonia loading was approximately 0.6 kg NH3-N/m3-day for the three BAFs of different media types. The zero-order biotransformation rates for the BAF with gravel were found to be higher than the lava rock and plastic ring media. The results ultimately showed that BAF can be used as an add-on system to aerated lagoons or as a secondary treatment unit to meet ammonia discharge limits.
In the United States Midwest, aerated lagoons are widely used by small communities for domestic wastewater treatment. Their wide spread use is credited to their relatively low capital costs and relatively simple operational and maintenance requirements. Nevertheless, most systems are designed for 5-day carbonaceous biochemical oxygen demand (CBOD5) and suspended solids removal, not ammonia removal. Over the past several years effluent ammonia limits have been added to the National Pollution Discharge Elimination System (NPDES) permits of these facilities. These aerated lagoons are able to meet the effluent ammonia concentration limits in the summer, but incapable during the late winter and early spring months, given the increase in effluent ammonia concentration from a decrease in nitrification activity over the winter months.
A survey of ten aerated lagoons in Iowa by Ong and co-workers indicated that some aerated lagoons perform better than others under identical climatic conditions [1]. Ong and co-workers concluded that the better performance of these facilities may be attributed to the longer hydraulic retention time (HRT) of the aerated lagoons. Providing longer retention times for aerated lagoons is not economical as a large area would be required. If HRT does indeed play an important role, then recirculation of the wastewater may improve nitrification. Just as important as the retention time is the maintenance of a large biomass of nitrifiers since nitrification is slow at low temperatures. Maintaining a large biomass is impractical for aerated lagoons due to their simple construction and innate hydraulics. A possible treatment unit process that combines both longer HRT and high biomass is the biological aerated filter (BAF). BAFs may be added as a treatment process after the aerated lagoons or used directly as a secondary treatment unit. Due to their compactness, BAFs have grown in popularity in Europe [2-4].
BAFs have a relatively low capital cost investment, are easy to operate, and are more efficient than activated sludge systems [5-8]. A BAF typically consists of a submerged, granular media that treats carbonaceous and nitrogenous matter using biomass fixed to the media and capturing the suspended solids in the media. Over the last two decades, different configurations have been developed to improve BAF treatment efficiency, including downflow [9] or upflow configurations [10-12] and use of various media. Pilot-scale studies seemed to suggest that BAFs might be effective in removing ammonia when the wastewater is recirculated [13]. Using an upflow BAF, a 90% ammonia removal was observed at an ammonia loading rate of 4.0 kg/m3-day, a hydraulic loading rate of 30 m3/m2-h, and a temperature of 17.5℃ [3]. The hydraulic loading rate used was approximately four times higher than the typical hydraulic loading rates for BAFs [3].
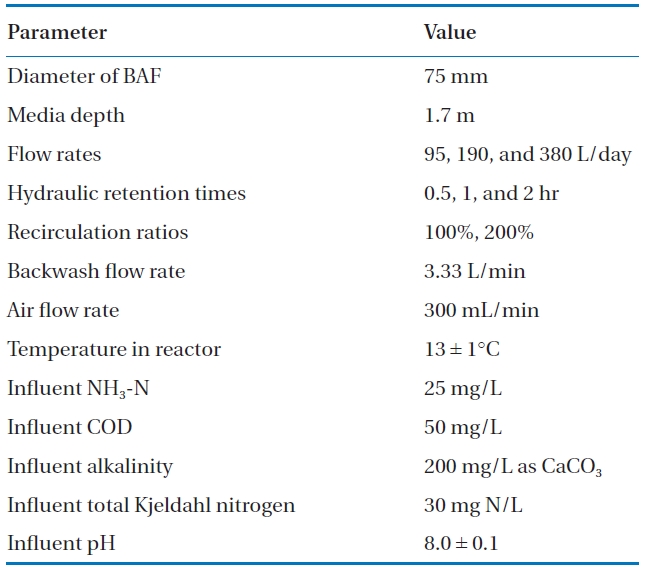
In a typical aerated lagoon system, the BAF can be located as an add-on system after the second cell of the aerated lagoon, thereby minimizing modification to the aerated lagoons. The objectives of this study are to investigate the application of BAFs using three different media types for nitrification under various operating conditions and provide a preliminary assessment of its feasibility for small communities in meeting their NPDES limits. The media selected had to be easily obtained and readily available. The subsequent BAFs were operated at various operating parameters, such as chemical oxygen demand (COD) and nitrogen loading rates, hydraulic loading rates, and effluent recirculation to ascertain effectiveness at an operating temperature of 13℃.
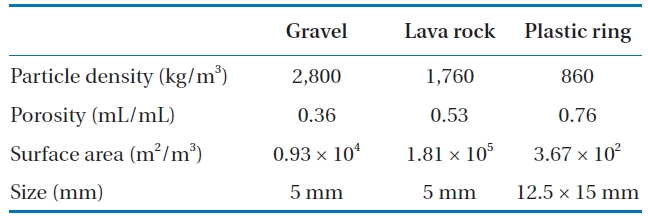
A schematic diagram of the pilot-scale BAF used in the experiments is shown in Fig. 1. Each BAF was constructed of a PVC pipe with a diameter of 75 mm and a media depth of 1.7 m. For each BAF, gravel, lava rock, and plastic rings were used as packing materials. The flow was in a down-flow mode. The size, bulk density, porosity, and specific surface areas for the gravel, lava lock, and plastic rings used are summarized in Table 1.
To assess BAF effectiveness under varying conditions, synthetic wastewater was used as feed wastewater (Table 2). The synthetic wastewater contained organic carbon, essential nutrients, ammonia, and minerals to simulate the characteristics of the effluent from aerated lagoons [1]. The COD and ammonia concentrations of the feed water were approximately 50 and 25 mg/L, respectively.
Peristaltic pumps (Masterflex Model 75530-70, Chicago, IL, USA) were used to feed synthetic wastewater to the BAFs, while other peristaltic pumps (Cole-Parmer 7553-50, Chicago, IL, USA) were employed for recirculation of the treated effluent through the filters. A centrifugal pump (Emerson Model S55JXDRL-2565, Chicago, IL, USA) provided backwashing. Filtered in-house compressed air was introduced into the BAF
[Table 1.] Physical properties of media
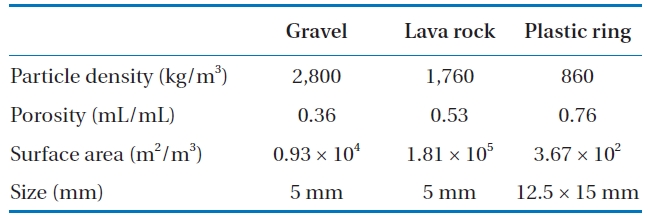
Physical properties of media
[Table 2.] Composition of synthetic wastewater
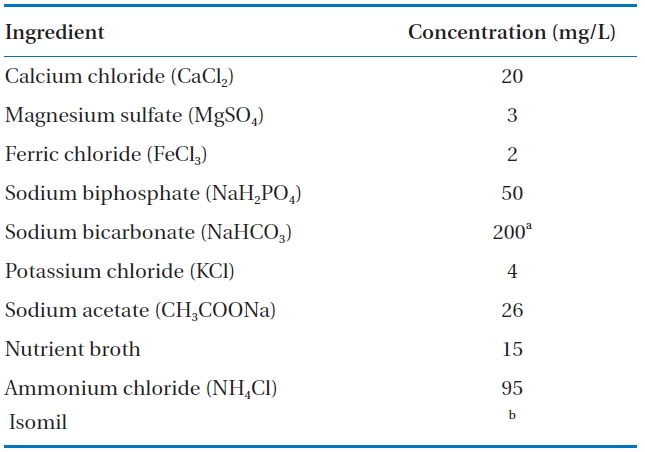
Composition of synthetic wastewater
The BAFs were seeded with activated sludge from an aeration tank of a municipal wastewater treatment plant in the state of Iowa. The three BAFs were initially operated at an HRT of two hours without recirculation at room temperature (24℃). When the soluble COD (sCOD) and ammonia percent removal exceeded 90%, the temperature of the synthetic wastewater was reduced to 13 ± 1℃, representing typical early spring conditions. At this temperature, the BAFs were operated at HRTs of two, one, and 0.5 hours without recirculation, followed by the same HRTs with recirculation at 100 or 200% of the influent flow rates. The nominal flow rates were 95, 190, and 380 L/day, giving hydraulic loadings ranging from 1.0 to 4.0 m3/m2-h. Linear velocities with in the columns for 2-, 1-, and 0.5-hour HRTs, as well as 1-hour HRT plus 100% recirculation, were 0.85, 1.7, 3.4, and 3.4 m/h, respectively. Backwashing of the BAFs were performed every two days for the 0.5-hour HRT, every four days for the 1-hour HRT, and once a week for the 2-hour HRT with 100 and 200% recirculation. For each HRT, the BAFs were operated over a two to three week period until the three consecutive effluent COD concentrations did not vary by more than 10%. Operating conditions of the BAFs are listed in Table 3.
The influent and effluent of the BAFs were sampled and analyzed for sCOD, total Kjeldahl nitrogen (TKN), alkalinity, ammonia, nitrate, and nitrite. Other parameters measured include pH, temperature, DO, and flow rates. Suspended solids (SS) in the effluent of the BAF were measured for the 2-hour HRT and found to be 6.0 ± 1.7 mg/L. Subsequent SS measurements were not conducted. Samples used for the analyses were one-hour composite samples. Analyses were conducted according to Standard Methods [14].
3.1. Impact of Media Type and HRT without Recirculation on Removal Efficiencies
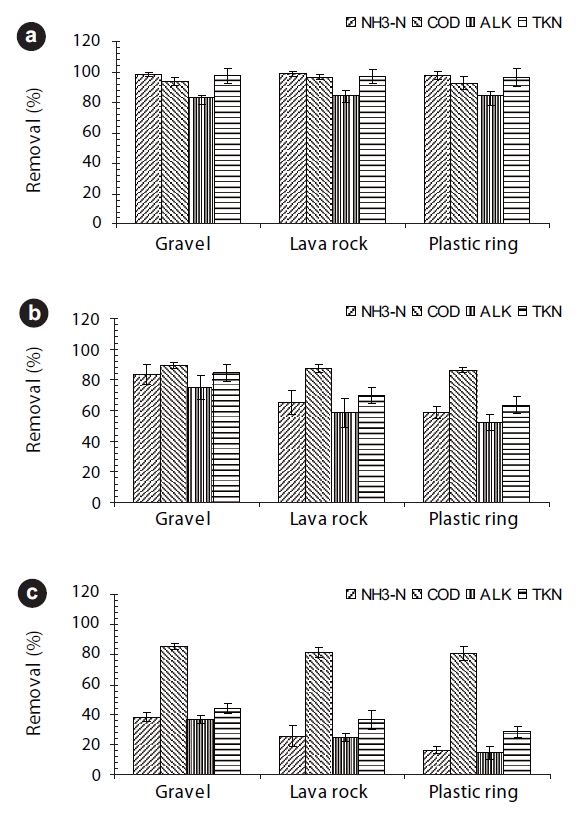
The average NH3-N, sCOD, TKN, and alkalinity percent removals (with 95% confidence intervals [CI]) by the BAFs for different media types and HRTs without recirculation are presented in Fig. 2. For an HRT of two hours, more than 97% of ammonia and 92% of the sCOD were removed for all three media types (Fig. 2a). Carbonaceous and nitrogenous matter was oxidized in the BAFs by the biomass attached to the submerged granular media. Meanwhile, suspended solids in the wastewater were simultaneously filtered or captured within the media. Ammonia was nitrified to nitrate by autotrophic microorganisms within the media. As indirect evidence of nitrification, more than 83% of the influent alkalinity was consumed. The average ratios of consumed alkalinity to oxidized ammonia were 6.7, 7.5, and 7.6 mg CaCO3/mg NH3-N for gravel, lava rock, and the plastic rings media, all reasonably close to the theoretical value of 7.14 mg CaCO3/mg NH3-N. TKN removals were approximately 97% for all three media types for the 2-hour HRT.
[Table 3.] Experimental operating conditions for biological aerated filters
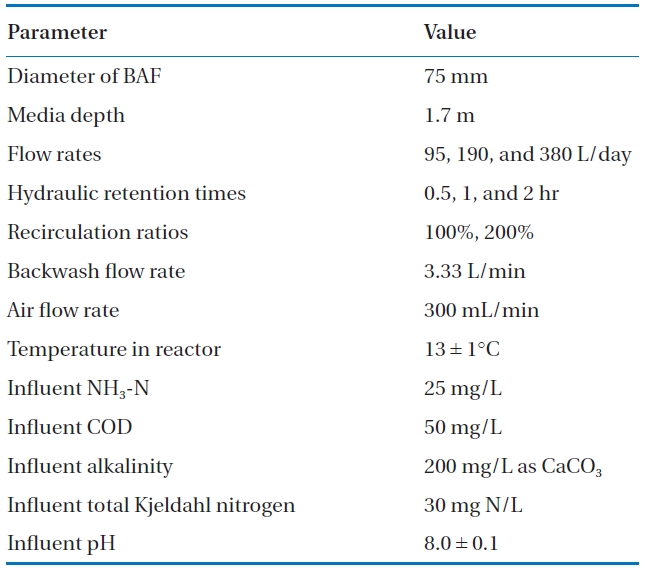
Experimental operating conditions for biological aerated filters
For the BAF run with a 1-hour HRT, approximately 83% of the ammonia was removed in the BAF by the gravel media, and 65% and 59% for the lava rock and plastic rings. The sCOD percent removals for all three media exceeded 85%. The BAF with the gravel media removed approximately 75% of the influent alkalinity and 84.5% of the influent TKN, both statistically different and higher than the percent removals for the lava rock and plastic rings BAFs (Fig. 2b).
For an HRT of 0.5 hour without recirculation, the percent removals for ammonia for all three media types were less than 40% (Fig. 2c). However, sCOD percent removals for all three BAFs were above 80%. The impact of the HRT on ammonia removal was apparent, with a reduction in percent removal of ammonia for a shorter HRT.
Based on the above results, an HRT of two hours without recirculation would be sufficient for all three media to obtain an ammonia concentration less than 1.0 mg/L for an influent ammonia concentration of 25.0 mg N/L and a sCOD of 50.0 mg/L at a wastewater temperature of 13℃. However, all three media types were unable to achieve an ammonia concentration of 1.0 mg N/L at an HRT of one hour without recirculation; however, the BAF with gravel media gave the lowest average ammonia effluent concentration compared to the other two media. A possible reason for the better performance of the gravel media BAF was the higher specific surface area for the biomass growth compared to the lava rock and plastic rings. It should nevertheless be noted that the lava rock had a tendency to break into smaller particles.
3.2. Impact of Recirculation on Ammonia and sCOD Removal
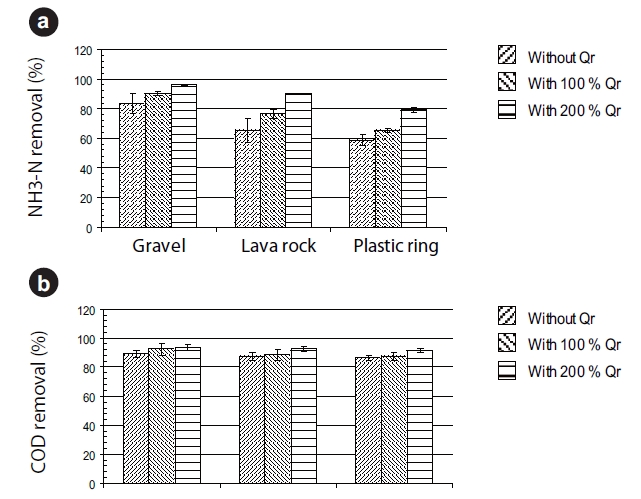
Since the percent removals for ammonia for all three media were greater than 90%, the impact of recirculation was generally conducted with an HRT of one hour. The results for 100 and 200% recirculation of the effluent wastewater and at one hour HRT on ammonia and sCOD percent removals are presented in Fig. 3. The results showed that ammonia percent removals improved from 83 to 90, 65 to 77, and 59 to 65% for gravel, lava rock, and plastic rings, respectively, when the BAF was operated with 100% recirculation. With 200% recirculation, ammonia removal improved further to 96, 90, and 80% for gravel, lava rock, and plastic rings, respectively. The sCOD removal with 200% recirculation at a 1-hour HRT was greater than 90% for all three media (Fig. 3b).
Several researchers observed moderate improvement in nitrification efficiency with an increase in water velocity by recirculation which has attributed the improvement to enhanced mass transfer of ammonia into the biofilms [3, 15, 16]. Another possible explanation is dilution of influent ammonia and sCOD from the recirculation of the effluent, resulting in a lower concentration of ammonia that could be transformed over a shorter HRT. Using a mass balance of ammonia, higher ammonia removal was not totally due to dilution. For the gravel media BAF, the effluent ammonia concentration was approximately 4.0 mg N/L for an HRT of one hour without recirculation. With 100% recirculation, the ammonia concentration entering the gravel media BAF would be approximately at 14.5 mg N/L, slightly higher than half of the influent ammonia concentration of 25.0 mg N/L. Assuming that the ammonia percent removal for 100% recirculation at a 1-hour HRT was similar to the BAF run at a 0.5-hour HRT without recirculation, nearly 40% ammonia removal (Fig. 2c), the estimated effluent ammonia concentration for 1-hour HRT and 100% recirculation, was approximately 8.7 mg N/L (14.5 mg N/L × 0.6). Since the experimental effluent ammonia concentration was 2.5 mg N/L, lower than the estimated 8.7 mg N/L, it is possible that recirculation had an impact on nitrification within the BAF even though the water linear velocity had doubled through the gravel media.
3.3. Changes in Ammonia and Nitrate Concentrations with Media
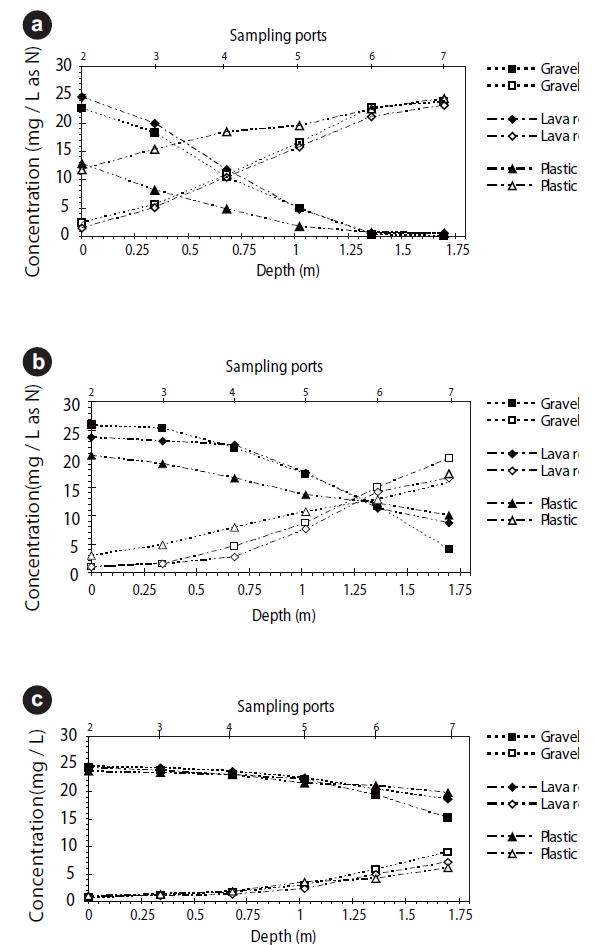
The changes in ammonia and nitrate concentrations within the bioreactor at different HRTs and media types without recirculation are presented in Fig. 4. Three different ammonia and COD loadings were applied by changing the HRTs, while maintaining the influent COD concentration at 50 mg/L and the ammonia concentration at 25 mg N/L.
With respect to the three media at an HRT of two hours (Fig. 4a), complete nitrification of ammonia was observed within the initial 1.4 m of the media. Appearance of nitrate within the media was in good agreement with the disappearance of ammonia (Fig. 4).
For 0.5-hour and 1-hour HRTs, significant differences in ammonia concentrations were observed within the depth of the media for all three media types. Negligible nitrification was observed within the first 0.7 m of all three columns for an HRT of 0.5 hour (Fig. 4c). This may be due to an increase in hydraulic loading, possibly resulting in an insufficient bed volume available for nitrification. Meanwhile, the nitrite concentrations increased to nearly 3.0 mg N/L within the media, but decreased to less than 1.0 mg N/L at the end of the media bed.
3.4. Overall Ammonia Biotransformation Rates in BAFs
The overall ammonia biotransformation rate (k) within the BAF may be modeled as a zero-order reaction with respect to ammonia concentration [17] and is given by Eq. (1) by assuming a simple input-output model and steady state conditions:
where
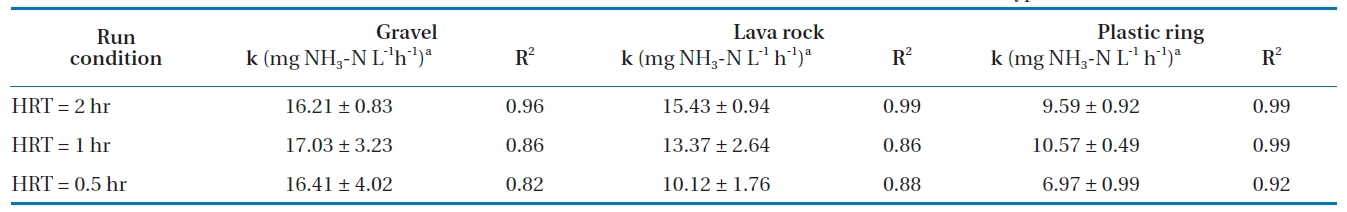
The estimated zero-order biotransformation rates, along with their 95% CI, are summarized in Table 4. The biotransformation rates in gravel at different HRTs were higher than the rates for lava rock and plastic rings, indicating that gravel may be a better material. The zero-order biotransformation rates for the gravel media were statistically similar for all three HRTs tested (2, 1, and 0.5 hours), confirming that nitrification occurred at the same rate, but the lower percent removal at a shorter HRT was due to insufficient retention time. For the lava rock media, the biotransformation rates were statistically different between the 2-hour and 0.5-hour HRTs, while the rates for the plastic rings were different for all three HRTs. This indicates that at a shorter HRT, biofilm shearing can occur, resulting in a lower biomass and therefore lower nitrification and biotransformation rates. Statistically, the zero-order biotransformation rates for lava rock for 1-hour and 2-hour HRTs were similar to that of the gravel media.
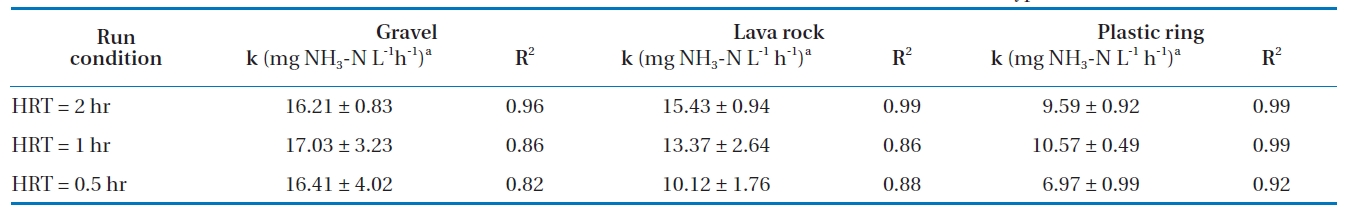
Estimation of zero-order ammonia biotransformation rates in the BAF for various HRTs and media types at 13 ± 1°
3.5. Impact of Ammonia and sCOD Loading
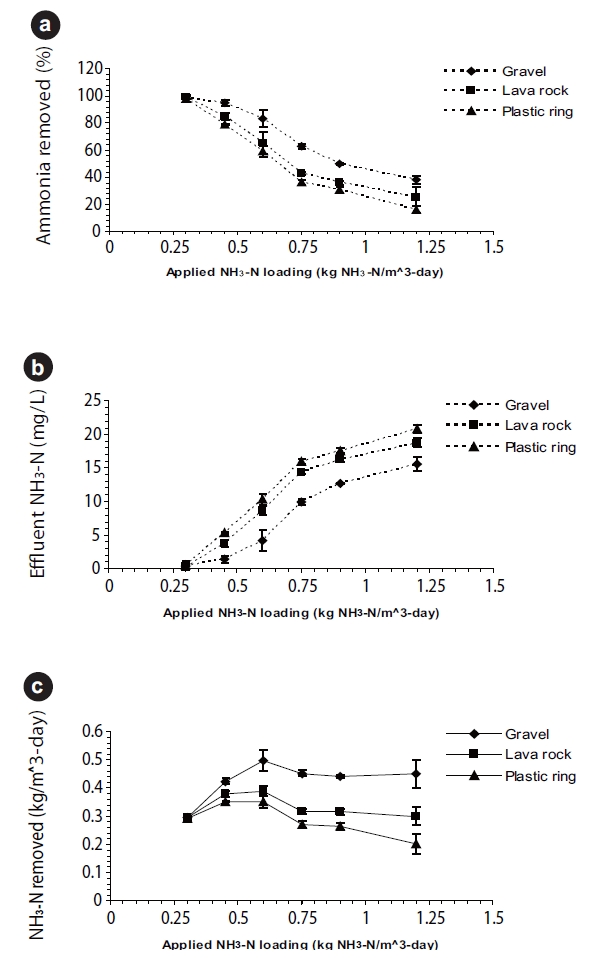
For the various experimental runs, the ammonia and sCOD loadings were plotted against the ammonia percent removals, effluent ammonia concentrations, and ammonia mass removed as shown in Fig. 5. For all three ammonia parameters, the ammonia percent removals decreased (Fig. 5a), while the effluent ammonia concentrations increased (Fig. 5b) with an increase in the ammonia and sCOD loadings. The ammonia mass removed for the three media were statistically different; for the gravel BAF, the ammonia mass removed increased to approximately 0.5 kg NH3-N/m3-day and remained fairly constant for a further increase in ammonia loading.
These observations suggest that the higher sCOD loading favored the heterotrophs over the autotrophs as indicated in the study where high CBOD5 loadings resulted in an increase in effluent ammonia concentration [18]. It is possible that a heterotrophic layer may be formed over the nitrifying biofilm, limiting the oxygen supply for the nitrifers [19].
Experimental results for three BAFs with different media showed that the BAF may be used as an add-on polishing unit to aerated lagoons or as a secondary treatment unit to meet ammonia discharge limits. Of the three media tested, gravel at a depth of 1.7 m gave greater than 96% nitrification for HRTs as low as 0.5 hours with 200% recirculation. Lava rock media was found to be unsuitable as the media broke easily to smaller particles. For the plastic rings, the lower specific surface area is likely the result of a lower biomass in the BAF, which in turn affected the amount of nitrification for shorter HRTs compared to the other two media types. Experimental results showed that ammonia percent removals at an HRT of one hour and a temperature of 13℃ improved significantly with 100 and 200% recirculation of the effluent. Based on the ammonia and COD loadings, a maximum ammonia loading of 0.6 kg NH3-N/m3-day was determined for all three media, after which an increase in the loading did not improve ammonia mass removed. The zero-order biotransformation rates in gravel were significantly similar at approximately 16.0 mg NH3-N/L-h for the three HRTs tested.
The project was funded by the U.S. Environmental Protection Agency, Cincinnati, Ohio under Contract No. CR-828754-01.