Polycyclic aromatic hydrocarbons (PAHs) are hydrophobic and composed of two or more fused aromatic rings. PAHs ex-ist in the natural environment, and originate from natural and anthropogenic substances. Industrial development has brought about increasing anthropogenic generation of PAHs. The diffuse sources of PAH-contamination are primarily the incomplete combustion of fossil fuels for heating, and the emissions from the incomplete combustion of fuels in the traffic sector. PAHs are widely distributed in soils and sediments, ground water and in the atmosphere. The persistence of PAH-contaminants in the environment affects humans directly and indirectly. PAHs are a serious risk to human health due to their carcinogenic potential. PAH exposure occurs by inhalation, ingestion and dermal contact. PAH metabolism in the human body produces epoxide compounds, with mutagenic and carcinogenic properties.
Bioremediation, which is also referred to as bioreclamation and biorestoration, is used to remove pollutants from the natural environment and/or convert the pollutants to less harmful products using the indigenous microbiological community of the contaminated environment [1]. It is commonly believed that in aged soils and sediments, the slow and/or incomplete biodegradation of petroleum hydrocarbons could be due to bioavailability limitations [2]. Hydrophobic organic compounds, such as PAHs and poylchlorinated biphenyls (PCBs), in soils, sediments and aquifers tend to be distributed among the solid, liquid and gas phases, such that only a small fraction of the contaminant mass may be present in the bulk water phase and available for biodegradation [3]. Al-Bashir et al. [4] reported that biodegradation was controlled by the rate of desorption when they investigated the behavior of nitrogen-substituted naphthalenes in flooded soil. Huesemann et al. [5] reported that the biodegradation of hydrocarbons is controlled by mass-transfer rate limitations. Carmichael et al. [6] also observed that the biodegradation kinetics in soils spiked with PAH were controlled by mass-transfer rate limitations. Moreover, it has been reported that the kinetic and extent of biologically-mediated PAH removal are associated with physical access by microorganisms to the molecules [7]. Since no study has been performed with respect to the mixing effects on the biodegradation of sorbed organic contaminants in a slurry system composed of heterogeneous materials, such as bacteria, water and solid, this study was initiated to determine whether different mixing methods can reduce these bioavailability limitations.
The main objective of this study was to evaluate the effect of four different mixing methods on the biodegradation of naphthalene (C10H8) and phenanthrene (C14H10) in soil slurry systems. The four mixing methods investigated were no mixing, orbital shaking, rolling and rotating.
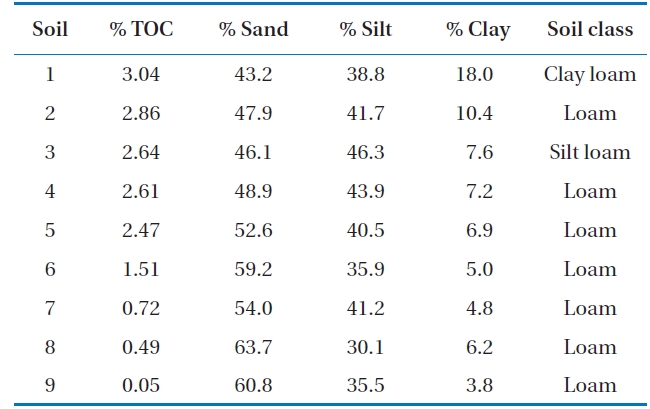
Soil samples were obtained from 9 different sites in Gwangju, Korea. The soils were collected at a depth of 0-15 cm, dried in the shade, and passed through a 2-mm size sieve. These soils were classified as loam, silt or clay loam (Table 1).
2.2. Soil Distribution Coefficient
This study evaluated mixing methods for each soil during biodegradation. Each soil was suspended in a phosphate buff-ered solution (PBS, 20 mM, pH 7) at a soil/solution ratio of 1:10 in 4.2 mL screw-cap glass vials, and contaminated with stock solutions of naphthalene (3,980 mg/L) and phenanthrene (3,140 mg/L). The initial liquid phase concentrations ranged from 0 to 8 ppm. The vials were rolled for 2 days in the dark. After mixing, each vial was centrifuged for 10 minutes at 3,000 rpm to separate the phases, with 1 mL of the supernatant sampled. The concentrations of contaminants in the supernatant were measured by high pressure liquid chromatography (HPLC). To determine the concentrations sorbed into the soil, the remaining supernatants were decanted and the soil contaminants were extracted with methanol for 24 hours. The 24 hours equilibrium time was selected based on previous studies [8, 9]. The concentrations of compounds sorbed into the soil were measured by HPLC.
2.3. Bacteria Growth and Biodegradation Experiment
Pseudomonas sp. KM1 was isolated from PAH-contaminated soil, and grown in 200 mL of mineral salt medium containing 0.2 g naphthalene as the carbon source. The mineral salt medium consisted of 3.4 g KH2PO4, 3.55 g Na2HPO4, 1g (NH4)2SO4, and 10 mL Hutner mineral base each per liter. The Hutner mineral base contained 10.0 g nitrilotriacetic acid, 14.45 g MgSO4, 3.335 g CaCl22H2O, 0.00925 g (NH4)6Mo7O244H2O, 0.099 g FeSO47H2O, and 50 mL stock salts solution per liter (2.5 g EDTA, 10.95 g ZnSO4·7H2O, 1,000 mL DI water). The liquid culture was incubated by shaking at 150 rpm and room temperature, with the cell growth monitored by measuring the absorbance at 600 nm with a UV spectrophotometer. To prepare the bacteria inocula, bacterial cells in the late log phase were centrifuged, the pellet washed with a sterile PBS (20 mM), and re-suspended in buffer solution. The washing step was repeated three times to ensure the removal of any remaining naphthalene in the growth media. After washing, the cell population was re-suspended to an optical density of 2.0 at 600 nm, which corresponded to 1.14 × 109CFU/mL.
[Table 1.] The properties of soils
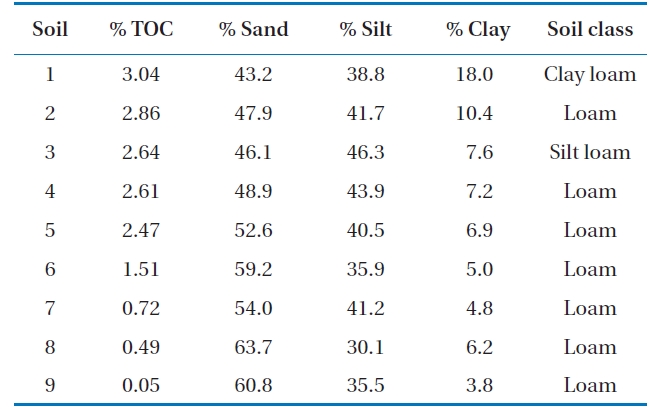
The properties of soils
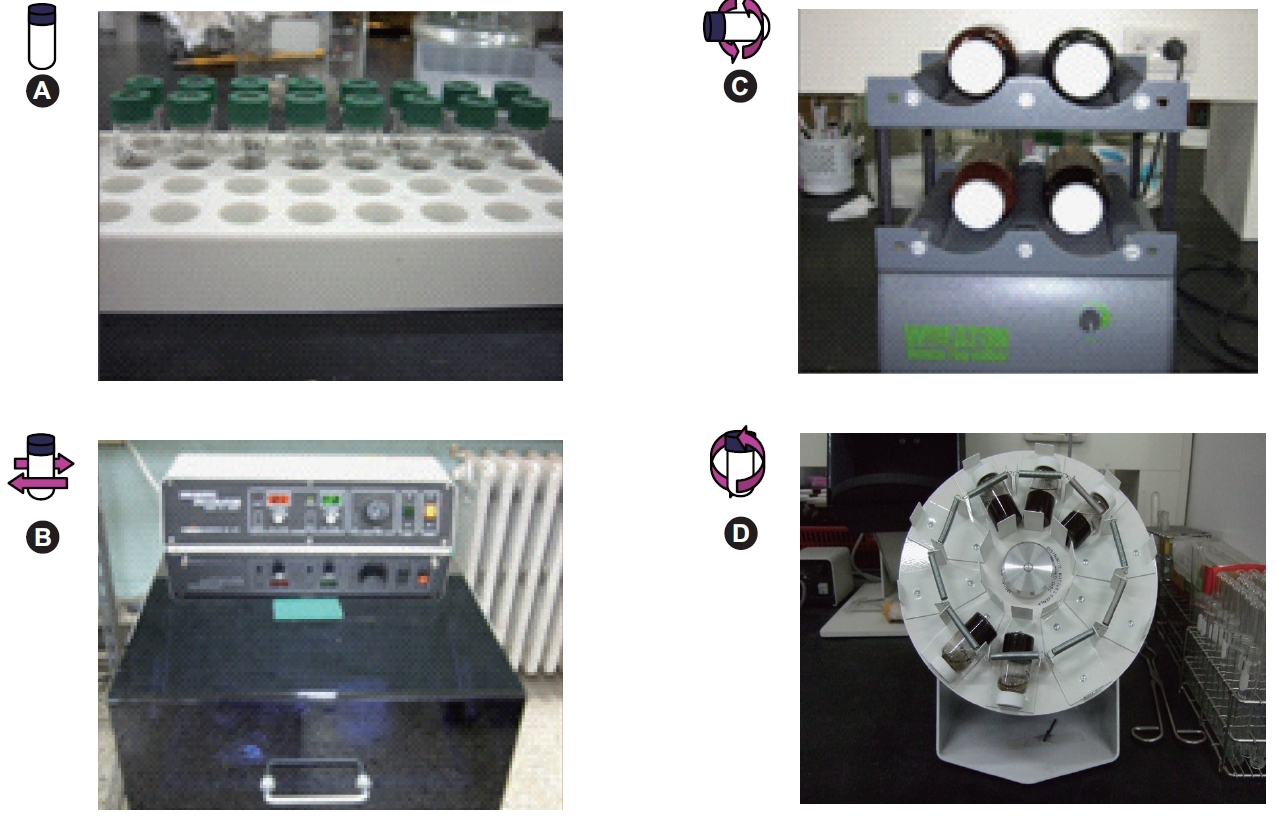
For the biodegradation experiment, each soil was suspended in PBS, with a soil to solution ratio of 1 to 10, and then contaminated with naphthalene and phenanthrene. The initial liquid phase concentrations of naphthalene and phenanthrene were 8 and 6 mg/L, respectively, in 4.2 mL screw-cap glass vials. The vials were rolled at 8 rpm for 1 day in the dark. After mixing, the vials were uncapped and 100 μL of the re-suspended cells introduced into each vial. To compare the effects of each mixing method, one series of samples were not mixed, the second series were mixed on an orbital shaking device at 150 rpm, the third series and fourth series were mixed at 8 rpm on a roller device and on a rotator machine, respectively (Fig. 1). With the rolling method, the vials were rolled and in the rotating method, the vials were tumbled up and down. After biodegradation for 1 and 3 days, the concentrations in the supernatant and sorbed phase were measured by HPLC.
2.4. Chemical Analysis
Naphthalene and phenanthrene were analyzed by HPLC using a LC-PAH column, with 80% acetonitrile/20% water as the mobile phase, at a flow rate of 0.5 mL/min, with UV detection at 254 nm.
Adsorption experiments were conducted for naphthalene and phenanthrene with 9 soils. The results showed that the re-lationship between the aqueous concentrations and the soil phase concentrations was nearly linear, with soil distribution coefficient (
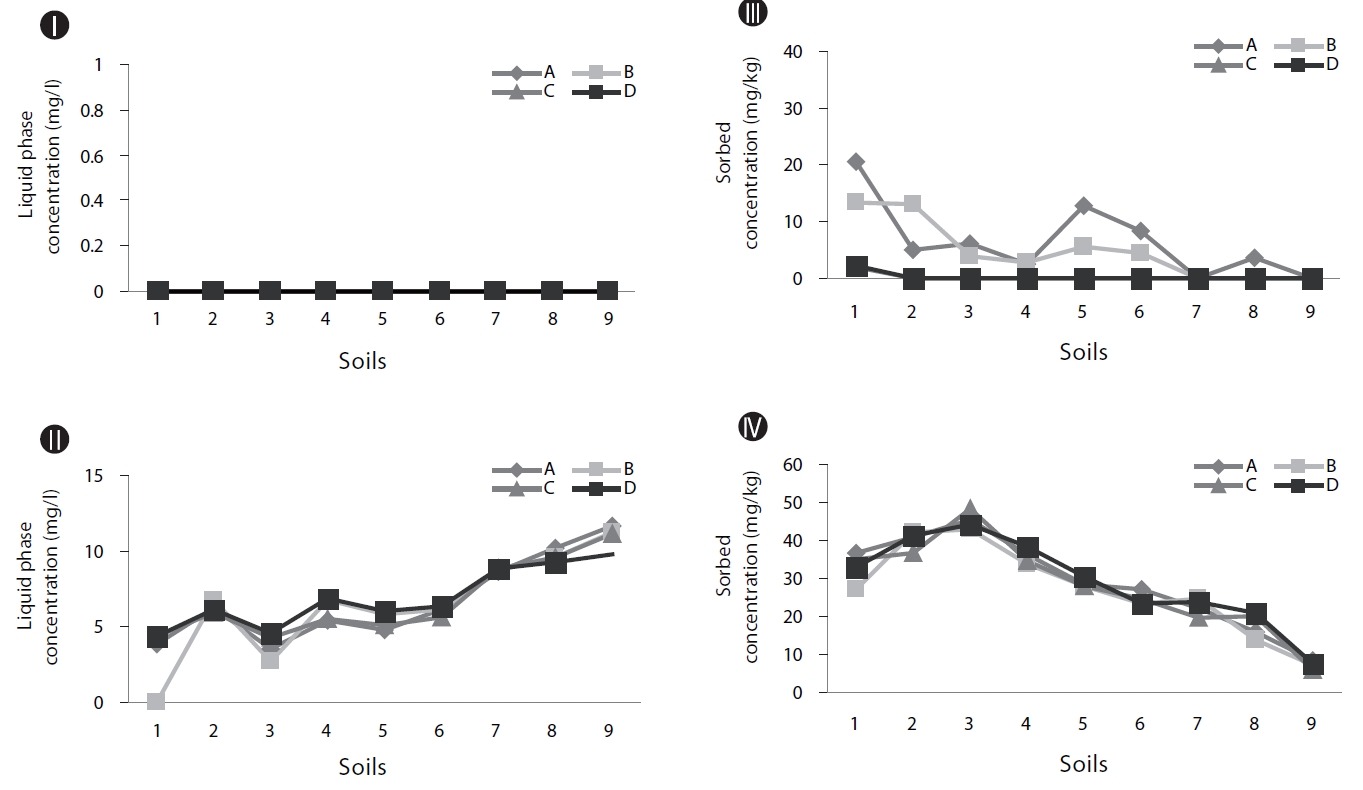
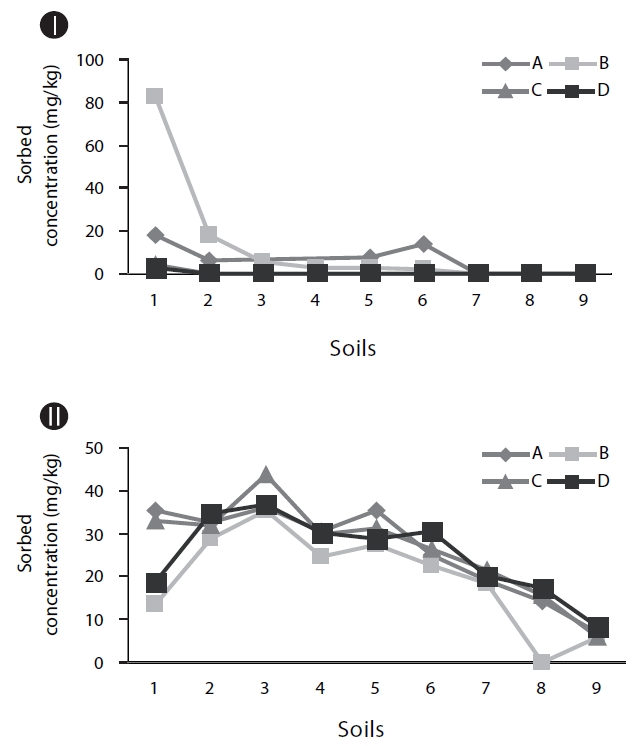
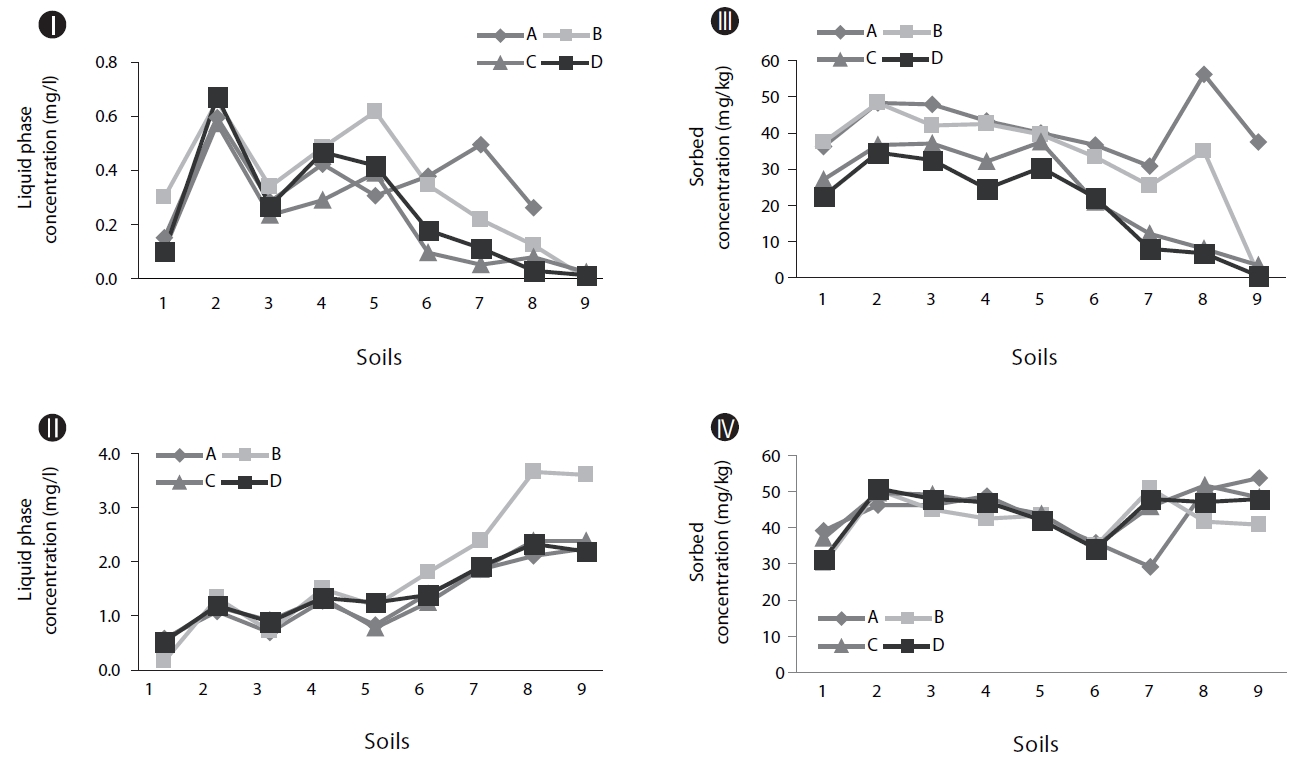
As shown in Figs. 2II and IV, without inoculum, the soils with higher OC values tend to show higher concentrations of naphthalene in the soil phase (Fig. 2IV) and lower concentrations in the supernatants (Fig. 2II). This suggests that OC plays an im-portant role in the retention of PAHs in soil. It has been reported that organic matter in soil plays the role of a strong sorbent in relation to PAHs, which significantly controls the partition of PAHs deeper into the soil profile [11].
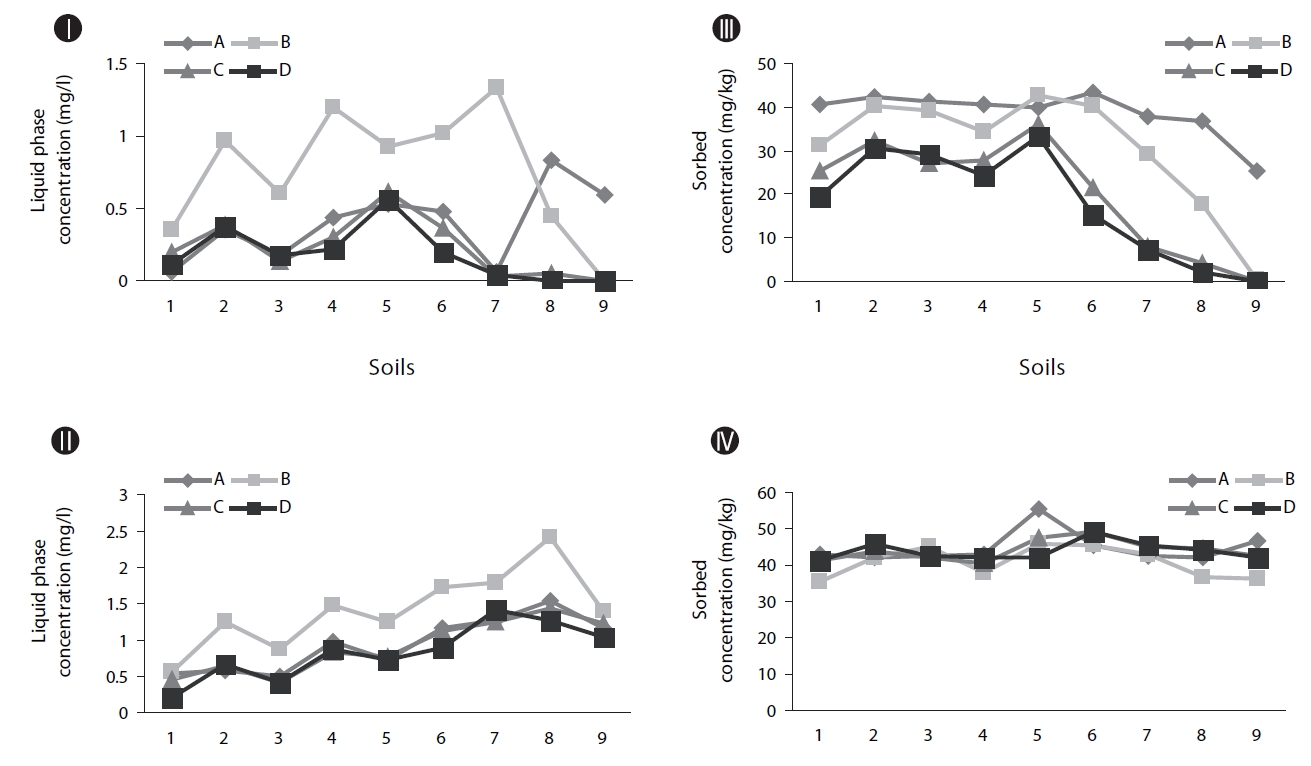
Biodegradation in soil slurry microcosms has been demonstrated in Figs. 2-5. With the biodegradation of naphthalene, most samples showed lower naphthalene concentrations in both the supernatant and soil with inocula compared to the concentrations in samples without inoculum (Figs. 2 and 3). The effects of the mixing methods on biodegradation of naphthalene are also demonstrated in Figs. 2 and 3 for 1 and 3 days, respectively. In Fig. 2III, the naphthalene concentrations sorbed to the samples with low OC contents (samples 7 through 9) were almost 0 mg/kg, regardless of the mixing method used. However, the type of mixing method was a significant factor in enhancing the biodegradation for samples with high OC contents (samples 1 through 6). The naphthalene concentrations sorbed were reduced to nearly 0 mg/kg via the rolling and rotating methods. However, with no mixing and the orbital shaking methods, the naphthalene concentrations sorbed were comparatively high, ranging from 2.59 to 20.45 mg/kg. The mixing methods are suggested to be an important factor for the biodegradation of contaminants sorbed in a slurry system composed of bacteria, liquid and solid. Conversely, the naphthalene concentrations sorbed into soils without microorganism inocula were similar for all four mixing methods (Fig. 2IV), which indicated there was no mixing effect on the sorbed concentration in the sorption experiment composed of liquid and solid. The mixing methods are suggested to not be a significant factor for the mass transfer of contaminants in a system without bacteria. As shown in Fig. 3, similar to the 1 day data, the naphthalene concentrations sorbed for 3 days were lower in the samples treated with the rolling and rotating methods with microorganism inocula. The mixing effect tended to be more significant in the system with soils with higher OC values (Figs. 2III and 3I).
Phenanthrene biodegradation demonstrated similar trends to that of naphthalene (Figs. 4 and 5). Figs. 4I and II showed the phenanthrene concentrations in the supernatant after 1 day of treatment. The phenanthrene concentrations in the supernatant with microorganism inocula were lower than without inoculum. Similarly, the phenanthrene concentrations sorbed with microorganism inocula in the soil phase were lower than without inoculum (Figs. 4III and IV). Moreover, the sorbedphenanthrene was more degraded when treated with the rolling and rotating methods with microorganism inoculum (Fig. 4III). The phenanthrene concentrations for 3 days showed similar trends to those samples treated for 1 day (Fig. 5). The phenanthrene concentrations sorbed into the soil phase with microorganism inocula were lower when treated with the rolling and rotating mixing methods (Fig. 5III).
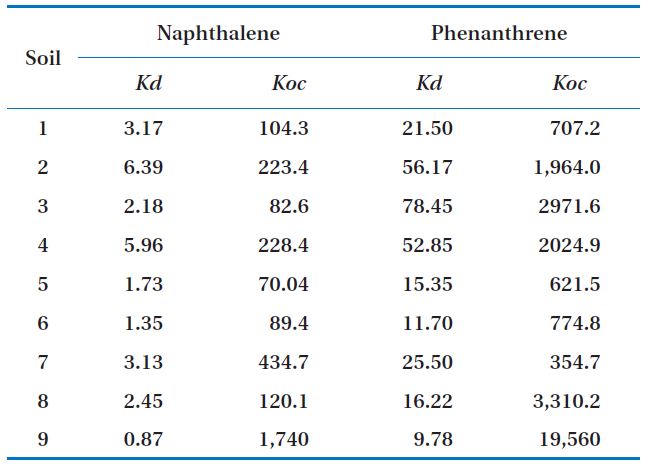
[Table 2.] Kd and Koc of soils
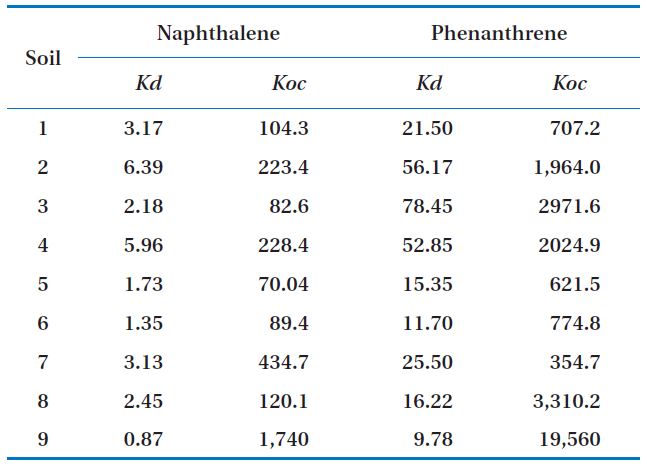
Kd and Koc of soils
Phenanthrene had higher Koc values for most soils, so its mobility was lower than that of naphthalene (Table 2). The phenanthrene concentrations sorbed into the soils were higher than those of naphthalene for all the soil samples. This may have been due to the higher adsorption coefficient and higher octanol-water partitioning coefficient values of phenanthrene compared to those of naphthalene.
Numerous investigations have shown that a very high removal efficiency of PAHs could be obtained from slurry phase reactors [12-16]. Moreover, with a slurry, the contact between microorganisms, contaminants, nutrients and oxygen is the prevailing factor for the removal of PAHs, with the rate potentially depending on the physical access by microorganisms to the molecules [7]. It has also been reported that microbial accessibility to PAHs can be enhanced when the soil aggregates are broken [7]. Moreover, the capability of diffusion through organic matter or soil micropores is one of the factors enhancing the bioavailability of PAHs[7]. In this study, differences were observed in the bioavailability of sorbed contaminants between the different mixing methods, where biodegradation of both naphthalene and phenanthrene in soils was greatly improved by the rolling and rotating methods. A biodegradation slurry system is composed of gravity-heterogeneous materials, including bacteria, liquid and solid. The gravities of bacteria, liquid and soil solid are around 1.1, 1.0, and above 1.6, respectively. With no-mixing and the orbital shaking methods, phase separation may occur between these gravity-heterogeneous materials, inducing a heterogeneous distribution of the materials, with mass transfer limitations, resulting in reduced bioavailability of the sorbed contaminants. An apparent heterogeneous distribution, such as bacteria with bubbles on top, liquid in the middle and soil at the bottom, was observed in the orbital shaking. However, the rolling and rotating methods provided a homogeneous distribution of the materials due to the up and down mixing. It was expected that an increased probability of physical contact between bacteria and soils and a reduced mass transfer distance would be induced in a homogeneous distribution system, which will improve the biodegradation rate and bioavailability of the sorbed contaminants.
In this study, the effects of mixing methods on the biodegradation of naphthalene and phenanthrenesorbed into soils were investigated. Four different mixing methods, including no mixing, orbital shaking, rolling and rotating were evaluated and compared. The results showed that the concentrations of sorbed naphthalene were reduced to 0 mg/kg in the rolling and rotating methods. However, with no mixing and the orbital shaking methods the concentrations of sorbed naphthalene were comparatively high, ranging from 2.59 to 20.45 mg/kg. Similar trends were observed for the biodegradation of phenanthrene. It is suggested that the rolling and rotating mixing methods provided homogeneous distribution of the materials by up and down mixing, which increased the probability of physical contact and rate of mass transfer between bacteria and sorbed contaminants in the soils, improving the rate of biodegradation and bioavailability of the sorbed contaminants. Therefore, it could be concluded that rolling and rotating were more effective mixing methods than no-mixing and orbital shaking for the biodegradation of organic contaminants sorbed into soil slurry systems.
This research was financially supported by the Ministry of Education, Science Technology (MEST) and the Korea Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Regional Innovation.