Bung Boraphet reservoir is a located in Nakhon Sawan Province, Northern Thailand. It was constructed in 1930 in an area of swamp at the confluence of the Nan River, which feeds into the Chao Phaya River. Bung Boraphet has high ecological and social economic value, particularly in its biodiversity according to the Office of Natural Resources and Environment Policy and Planning[1].
Bung Boraphet reservoir is a multiuse water body. It currently serves 30,000 people living around the reservoir, and has been used for water supply, flood control, waste disposal, fisheries, aquaculture, farming, etc. Heavy metals in Bung Boraphet originate from natural and artificial sources. Natural heavy metals introduced into the reservoir come primarily from such sources as soil erosion, and dissolution of water soluble salts from the Nan Rivers. The distribution of heavy metals in the sediment of Bung Boraphet between January and April 1993 was examined by Petpiroon et al.[2]. The zinc (Zn), lead (Pb), and cadmium (Cd) concentrations were at normal levels of 18.51, 12.99, and 0.12 μg g-1, respectively. However, Pb was the only metal that showed an increase in concentration. Cu at 23.50 μg-1 was slightly higher than the normal level for earth sediment[2].
The benthos including aquatic snails, mussels, leeches, aquatic worms, and aquatic insects is an aggregation of organisms living on or at the bottom of a body of water. Mussels are filter feeders that live in the sea, lake or river bottom sediment for a few years. Therefore, mussels are commonly used as a monitoring organism. In general, the use of mussels as bioaccumulators of toxic metals has concentrated on analyses of their soft tissues. A previous study reported the presence of
The study examined the water quality including physical, chemical properties and to determine the concentration of heavy metals Cu and Pb in sediments, water, and tissues of
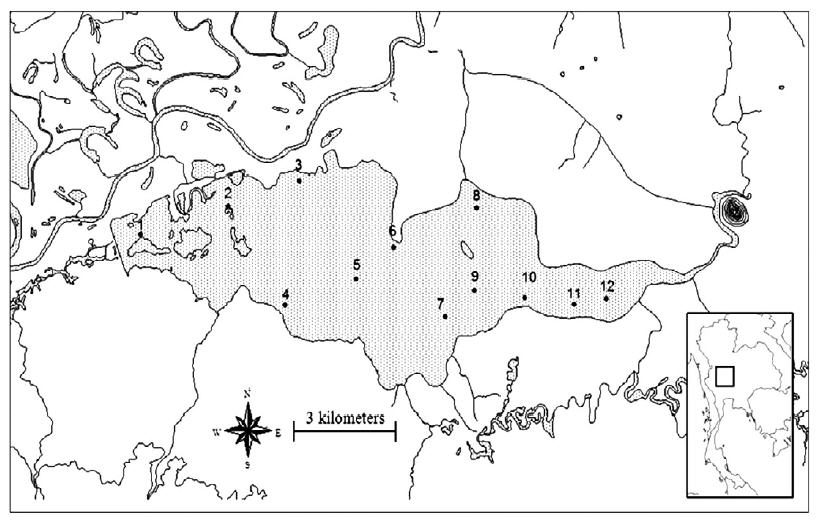
Bung Boraphet Reservoir is located between latitude 15°40′- 15°45′N and longitude 100°10′-100°23′E (approximately 20 m- >;meters above sea level) Nakhon Sawan Province, Thailand. The reservoir covers approximately 212.38 km2. The average water depth is 1.6 meters (maximum of water depth is 5 meters). The reservoir turns into a grass-covered plain with scattered ponds and swampy depressions during the dry season. In this study, water, sediment and Corbicula sp. samples were collected every 2 months from 12 study sites around Bung Boraphet (Fig. 1) between February and December 2008.
2.2. Investigation of Water Quality in Bung Boraphet Reservoir
The water physicochemical parameters including temperature, pH, turbidity, ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N), orthophosphates (PO3-4), biochemical oxygen demand (BOD), dissolved oxygen (DO), Cu, and Pb were examined according to the method reported by Eaton et al.[5].
Water samples were collected manually in polyethylene bottles. All bottles were cleaned with 10% HNO3 and rinsed with distilled water prior to use. All water samples were collected at approximately 30 cm beneath the water surface prior to transfer directly to the bottles. In the preservation stage, after sedimentation, concentrated nitric acid was added to adjust the pH to >; 2.
Sediment samples were collected using a specially modified Smith-McIntyre grab made from high grade stainless steel, while offshore samples were collected using a core sampler.
The
The clam samples were washed gently with deionized water to remove any sediment particles retained in the gills and mantle cavity. The clams were dried at 40-50℃, according to the method reported by Barsyte Lovejoy[6]. Soft tissues of 5 to 10 individuals were removed from the shell prior to homogenization using a mortar according to the method reported by Jeffrey et al.[7]. Sediment samples were dried at 100℃ in a hot air oven for 24 hours[8]. The dried sediment samples were crushed with an agate mortar. Depending on the availability of material, the homogenates were separated into two or three sub-samples for replicate analyses. The homogenized
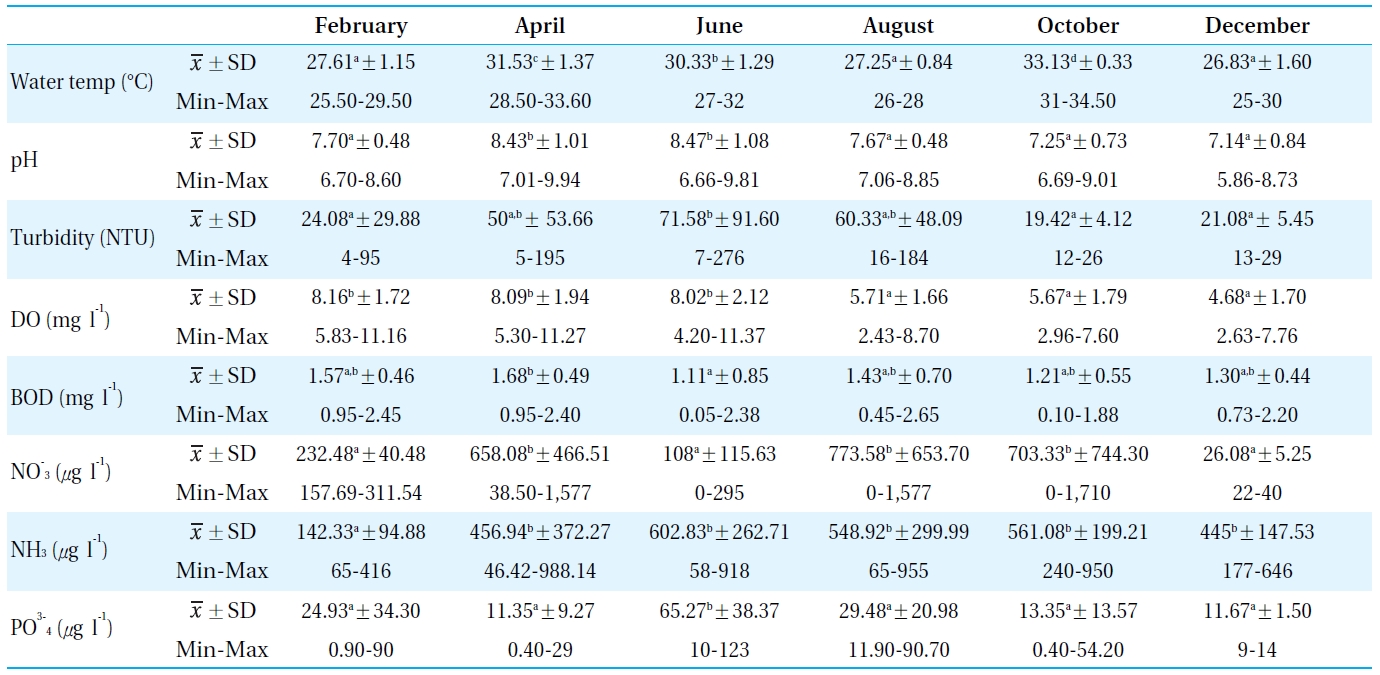
[Table 1.] Physiochemical parameters measured in the sampling sites from Bung Boraphet in 2008
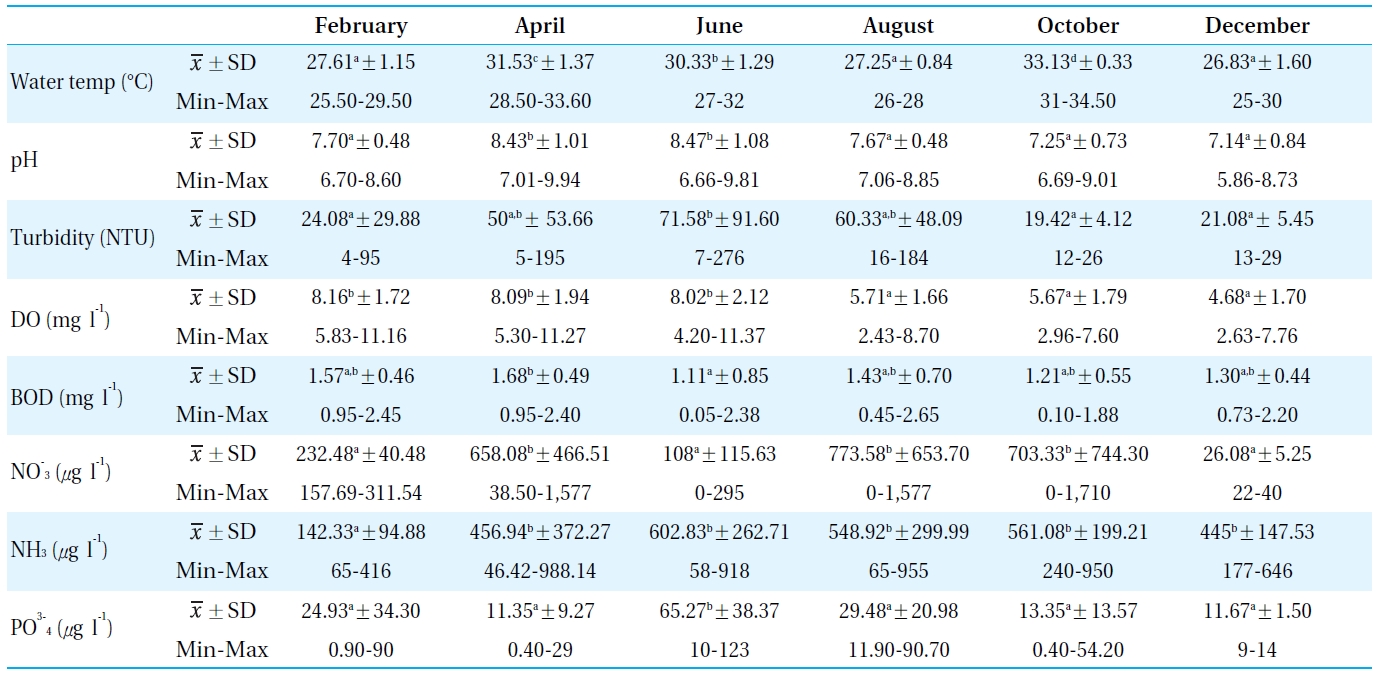
Physiochemical parameters measured in the sampling sites from Bung Boraphet in 2008
The mean (X) and standard deviations (SD) of each study sites were calculated. The results were evaluated for the statistical difference due to the extended exposure periods between the 6 months. The effects of seasonal changes in water quality and concentration of heavy metals were evaluated using a one way analysis of variance (ANOVA) and Post Hoc. Duncan test (
3.1. Water Quality in Bung Boraphet Reservoir
Table 1 lists the physicochemical parameters of the water in Bung Boraphet between February and December 2008. The water temperature, pH, turbidity, DO, BOD, NH3-N, NO3-N, and PO4-P were compared with the surface water quality standards of Thailand[11]. The assessment revealed the water quality of Bung Boraphet to be medium clean. It is suitable for human consumption and industrial purposes under sanitary controlled processes as well as agriculture. The water quality in this study was better than previous reported in 2006 by the Pollution Control Department[12]. The water quality was found to be poorer in the dry season than in the rainy season.
3.2. Distribution Study of Cu and Pb in Water Sediment and Tissues of Corbicula sp.
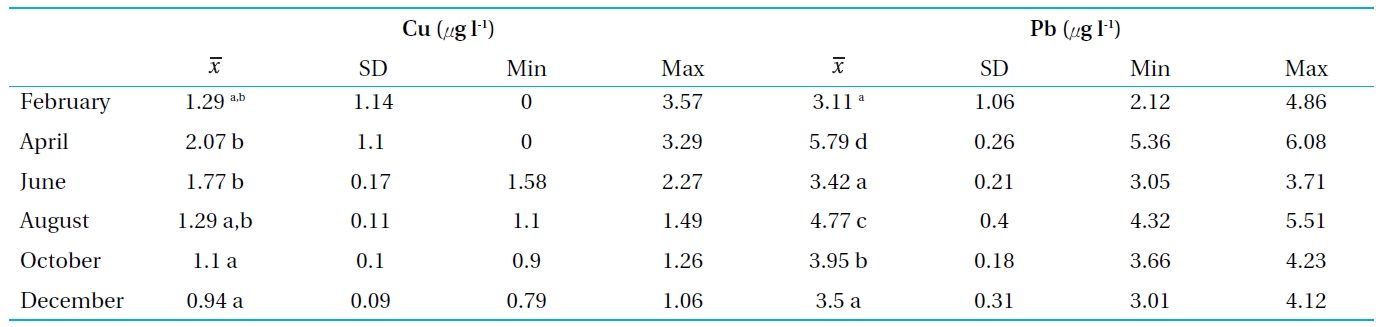
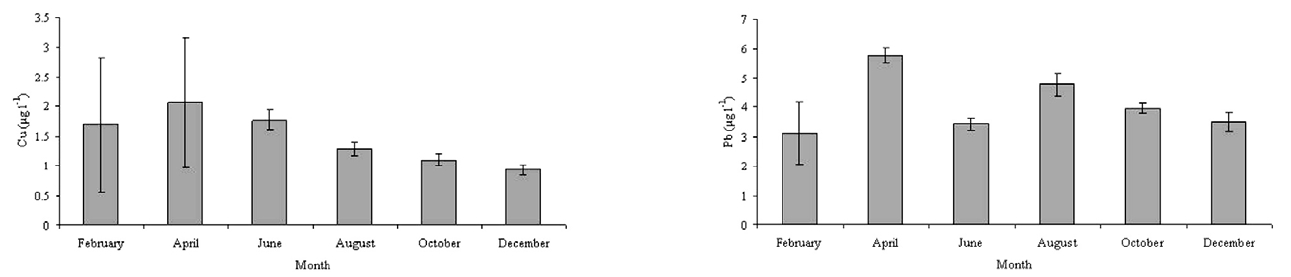
he concentration of heavy metals in water and sediments are presented in Tables 2 and 3 and Figs. 2 and 3. The levels od Cu and Pb were generally higher in the sediment than the clams and water, respectively. The highest concentration of Cu in the water of Bung Boraphet was observed in April (>; 2 μg l-1). The concentrations of Cu were <2 μg l-1 in February, June, August, and October, but <1 μg l-1 in December. The highest Pb concentration (<5 μg l-1) in water was detected in April while >;5 μg l-1 was detected in August. The same Pb concentrations of >;4 μg l-1 were observed in February, June, October, and December. The levels of Cu and Pb were satisfactory according to surface water quality standards of Thailand (≤0.1 and 0.05 mg l-1 for Cu and Pb, respectively)[11].
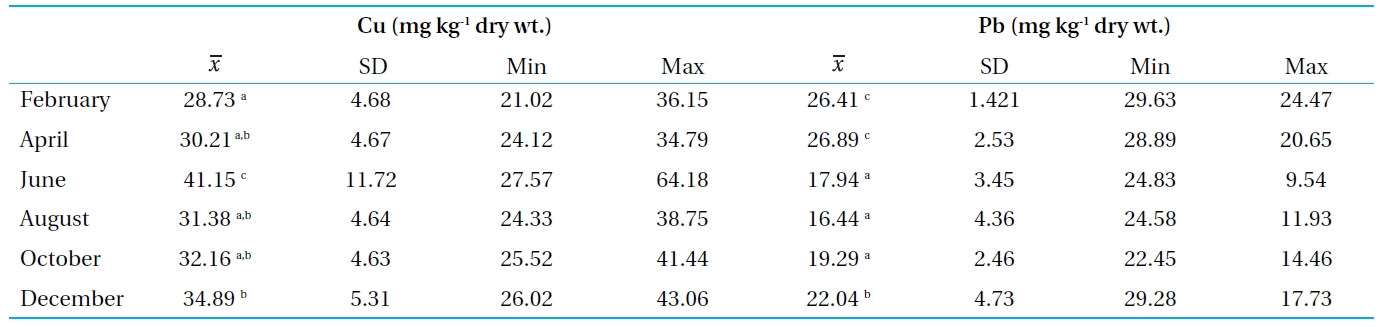
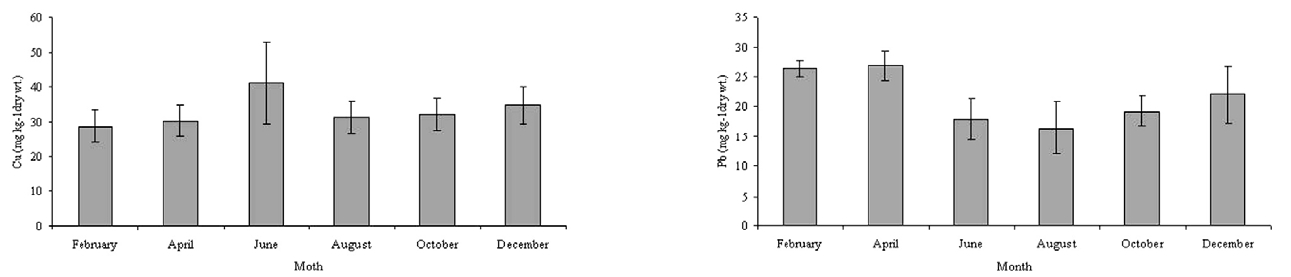
The Cu and Pb concentrations in the sediments were between 28.73-41.15 mg kg-1 and 16.44-26.89 mg kg-1, respectively. There is no standard established for the bottom sediments in Thailand. Therefore, the concentrations of the metals in the Bung Boraphet sediments were compared with the recommended lowest levels for Canadian sediment quality guidelines for the protection of aquatic life (≤35.7 and 35 mg kg-1 dry wt. for Cu and Pb, respectively)[13]. However, the concentrations of Cu in the sediments exceed the Canadian standard in June.
Higher concentrations of heavy metals in the sediment than in water were observed in this and other studies[6, 14-16]. Sediment is the major repository of metals, in some cases, holding more than 99 percent of the total amount of metal present in an aquatic system[17]. The observed high concentrations of Cu and Pb in this present study are consistent with previous findings[2]. These high Cu and Pb concentrations in the sediment can also cause metal pollution in the swamp.
[Table 2.] Concentrations of Cu and Pb in the water from Bung Boraphet
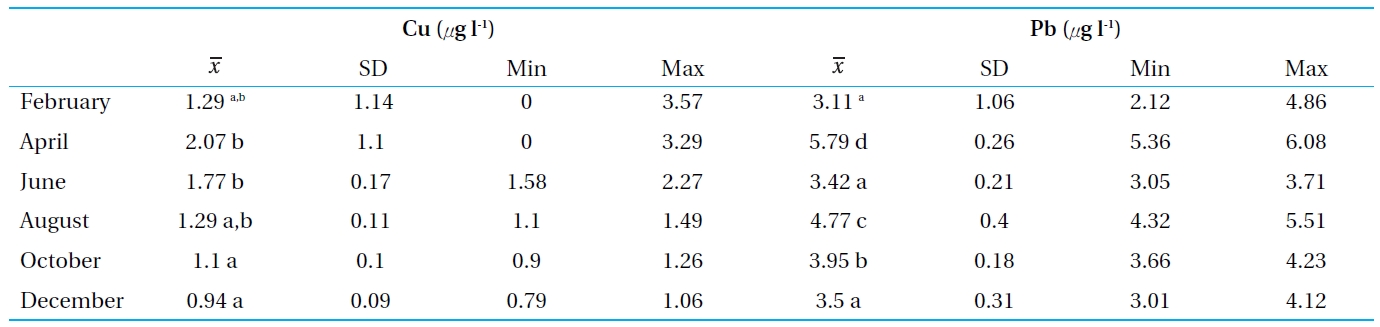
Concentrations of Cu and Pb in the water from Bung Boraphet
[Table 3.] Concentrations of Cu and Pb in sediment from Bung Boraphet
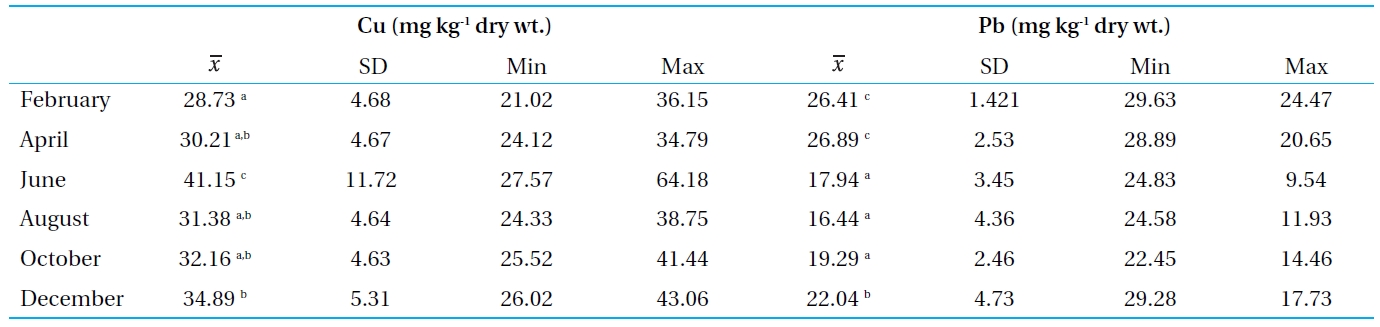
Concentrations of Cu and Pb in sediment from Bung Boraphet
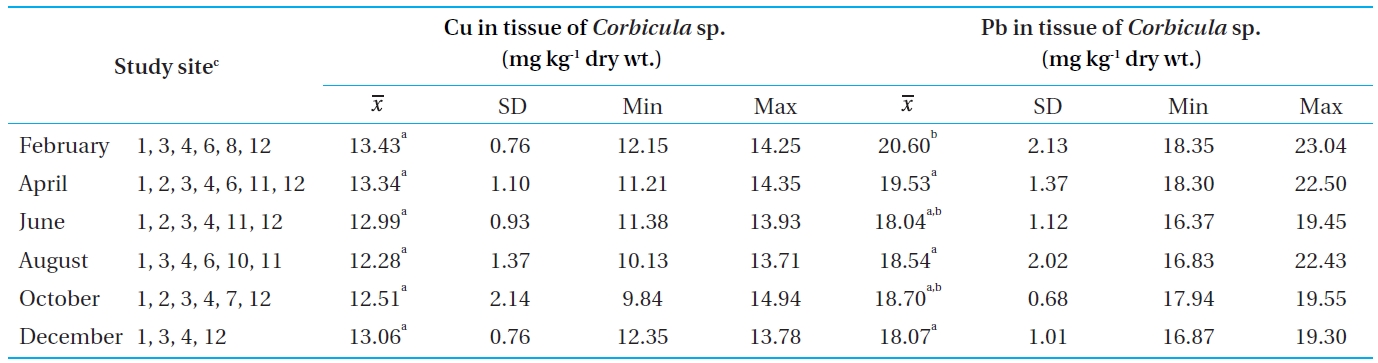
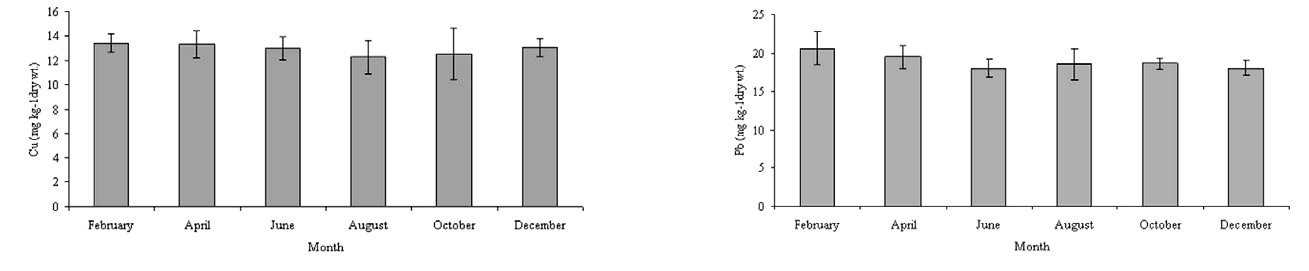
[Table 4.] Concentrations of Cu and Pb in the tissue of Corbicula sp. from Bung Boraphet
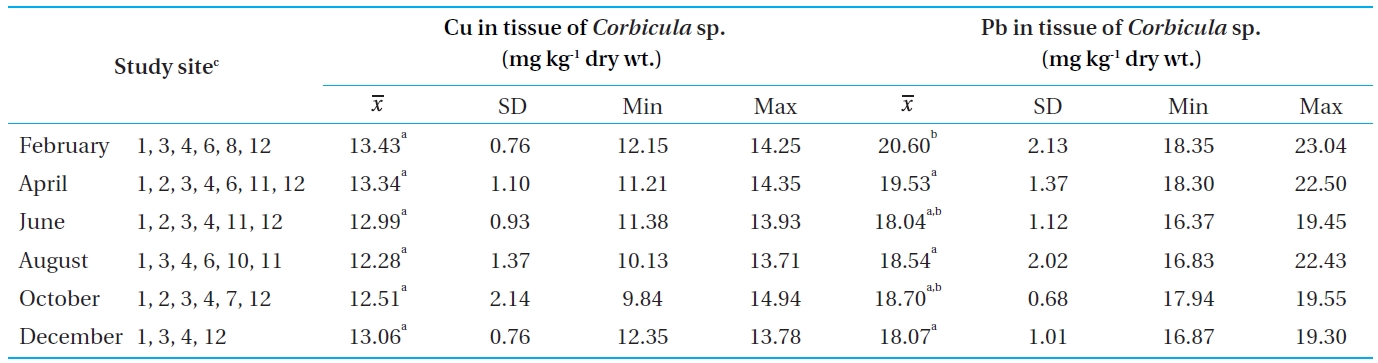
Concentrations of Cu and Pb in the tissue of Corbicula sp. from Bung Boraphet
The concentrations of Cu and Pb in tissues of
In this study, the Cu concentrations in the tissues of
This study examined the concentrations of Cu and Pb in water, sediment and
Financial support from Commission on Higher Education of Thailand, Centre for Environmental Health, Toxicology and Management of Chemical (ETM) and Graduate School, Chiang Mai University are gratefully acknowledged.