Silver nanoparticles (AgNPs) have a wide range of current and potential future applications, including spectrally selective coatings for solar energy absorption, chemical catalysts, surface-enhanced Raman scattering for imaging and; in particular, antimicrobial sterilization, which has made them one of the most commonly used nanomaterials[1-5]. Widely used NPs, such as AgNPs, will most likely enter the environment, and may produce a physiological response in certain organisms, possibly altering their fitness, and might ultimately change their populations or community densities. Research and literature regarding the ecotoxicity of NPs is still emerging, and gaps still exist in our knowledge of this area.
Despite the dramatic increase in the use of such nanomaterials, little information is available on their potential harmful effects on the environment. Most current literature on the toxicity of nanoparticles; however, comes from mammalian studies that have focused on respiratory exposure, or from
Genotoxic assessments of AgNPs were conducted on aquatic sentinel species, the freshwater crustacean
2.1. Organism Culture, AgNPs, and Ag Ion Preparation and Exposure to D. magna
Using an original strain provided by the Korea Institute of Toxicology (Daejeon, Korea),
2.2. Characterization of AgNPs
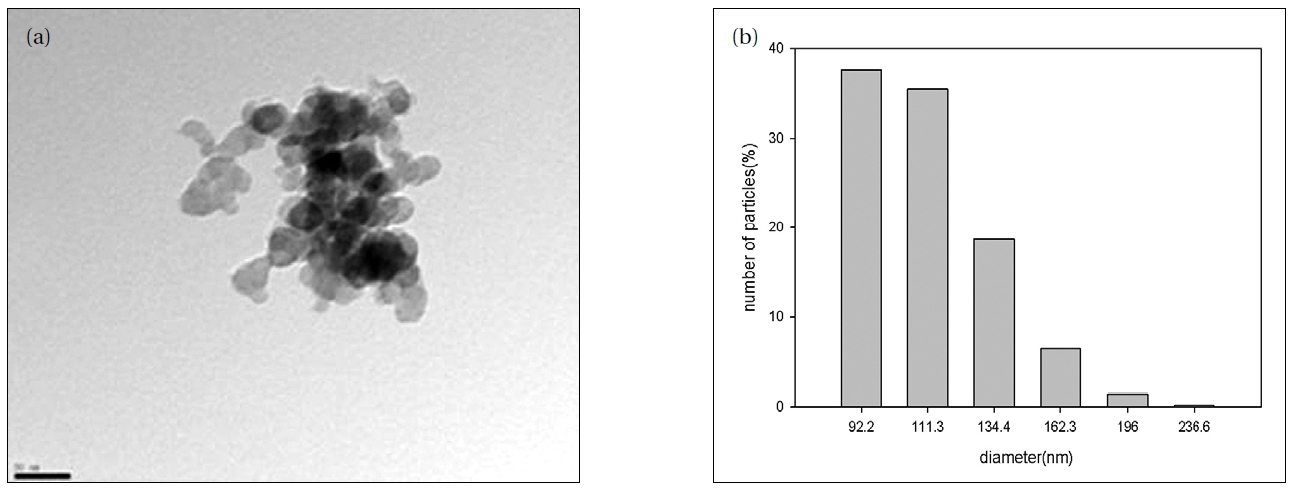
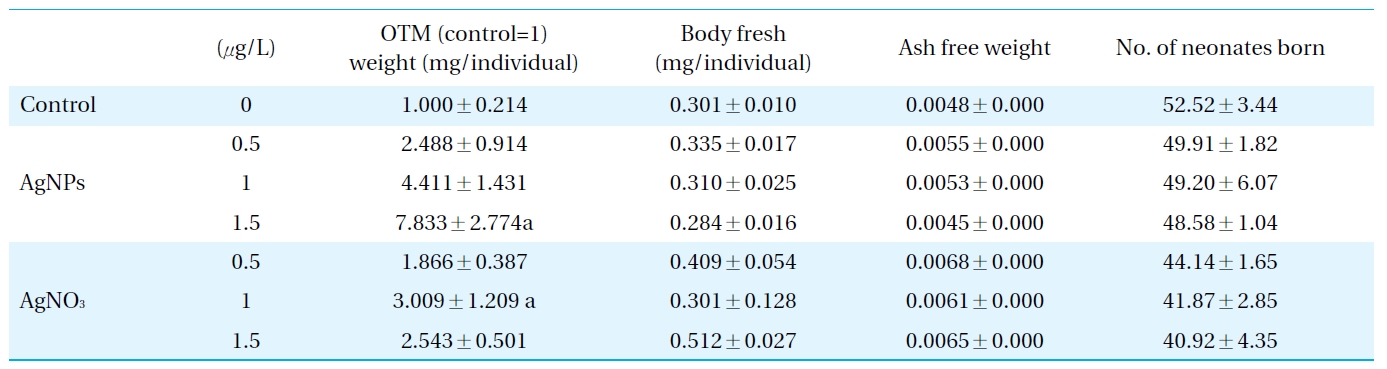
Energy filtering transmission electron microscopy (TEM) was used to examine the particle shape and size of the AgNPs. Twenty μL of the particle suspension were dried onto a 400 mesh carboncoated copper grid and imaged with a LIBRA 120 TEM (Carl Zeiss, Oberkochen, Baden-W rttemberg, Germany) at 80-120 kV. The size distribution of the AgNPs was evaluated using a photal dynamic light scattering (DLS) spectrometer, DLS-7000 (Otsuka Electronics Co., Inc., Osaka, Japan).
2.3. Mortality, Growth, Reproduction Assays
For the mortality test, 10 individuals, less than 24 hours, were exposed to AgNPs and Ag ions for 24 hours, with live and dead individuals then counted[23]. For the growth test, 20 individuals, less than 24 hours, were incubated with AgNPs and Ag ions for 96 hours, with the fresh weights measured immediately after exposure. The body dry weight was evaluated after drying
To prepare
The genotoxic- and ecotoxic assays results were tested for significance using an analysis of variance (ANOVA) test, employing the Dunnett's multiple comparison test. All statistical tests were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
The AgNPs used for the toxicity assays were characterized using TEM and DLS methods (Fig. 1). The TEM provided information on the size and shape of the nanoparticles, and showed sizes mainly <50 nm (Fig. 1a); however, it could not provide information on whether the nanoparticles existed in single or aggregated forms in the test medium, as the nanoparticles form aggregates when dried on the microscopic observation slide. The results of the DLS suggested that the AgNPs did not exist as single particles, but tended to aggregate in the test medium, as the main nanoparticle sizes distributed in the test medium were about 100 nm (Fig. 1b). In relation to nanotoxicity, it is often expected that the smaller the size, the stronger the toxicity exerted[26]. However, the relationship between the physico-chemical properties of nanoparticle and their toxicities seems to be much more complicated than just being related to their size and surface area (i.e. shape, charge, concentration, etc.); there is still much on going debate[8, 27, 28]. Many studies have failed to show any clear relationship between toxicity and the size of nanoparticles [8, 29]. The TEM images of the nanoparticles from the test medium showed the size of the nanoparticles tested. However, the line of evidence provided from the present study is rather limited; therefore, to identify key properties of nanoparticles with respect to causing ecotoxicity, toxic responses of a broad range of physico-chemical properties to various classes of nanoparticles may be investigated in various environmental relevant species.
[Table 1.] Acute toxicity of silver nano particles estimated in Daphnia magna

Acute toxicity of silver nano particles estimated in Daphnia magna
To find the range of sublethal concentrations for geno-and ecotoxicity tests, an acute toxicity test was performed on
DNA damage, particularly DNA strand breaks, was measured using the Comet assay to evaluate whether AgNPs induced any genetic toxicity in
Therefore, conventional ecotoxicity tests, using mortality, growth and reproduction as endpoints, were subsequently conducted to validate the ecotoxicological relevance of the response of DNA to damage in D. magna exposed to AgNPs and Ag ions (Table 2). The response of Daphnia to AgNPs in terms of their mortality, growth and reproduction may explain the higher biological-level consequences of the observed DNA damage. Aquatic toxicity tests may provide insights to the relative sensitivity of D. magna to AgNPs, which may also provide information on the impact of nanoparticles on water systems, as these species hold important positions in aquatic ecosystems[24, 31-33]. A significant increase in mortality was observed in D. magna exposed to 1.5 ㎕/L of AgNPs; whereas, no significant alteration was observed in growth and reproduction. It seemed Ag ion exposure leads to a slight increase in mortality, but decrease in reproduction potential; however, those alterations were not statistically significant. An increase in DNA strand breaks occurred concomitantly with an increase in mortality in D. magna exposed to 1.5 ㎕/L of AgNPs, which suggests DNA alteration induced by AgNPs might provoke higher level consequences. As mortality is the most obvious sign of progression of serious toxicity at the organism level, the impairment of survival due to AgNPs exposure may be considered a consequence of a serious progression of sub-organism level toxicities, such as the increased DNA damage in Daphnia. The relationships between the responses of the genotoxic biomarker and the physiological /individual/population effects are complicated due to the compensatory mechanisms regulating the physiological/ individual fitness and population dynamics in a natural system. As the mere presence of genotoxic compounds, which are potentially carcinogenic, is of major concern in human and ecosystem health, the sensitive and rapid detection of the genotoxic properties of aquatic systems themselves is considered important, although does not necessarily include alteration at a higher level of biological organization. Especially for the nanomaterials concerned, despite the dramatic increase in the use of nanomaterials and; hence, their ubiquitous distribution in aquatic environments, little information is available on their potential genotoxicity on aquatic organisms. Considering the potential of D. magna as a bioindicator species, and the importance of the genotoxicity of nanoparticles in ecotoxicity monitoring, the measurement of the DNA damage in these species after exposure to nanoparticles could provide useful information for freshwater monitoring. There have been discussions regarding the comparative toxicity of AgNPs and Ag ions[34, 35], with the latter’s bactericidal action having been studied previously[36, 37]. Our previous ecotoxicity study using Caenorhabditis elegans, comparing the toxicity of AgNPs and Ag ions, suggested that AgNPs were slightly more toxic than Ag ions in terms of their effect on reproduction potential, and it also appeared that different mechanisms exerted the toxicity of AgNPs and with Ag ions[38]. Results of the geno- and ecotoxicities (Table 2) in D. magna exposed to AgNPs and Ag ions also suggest that AgNPs are slightly more toxic than Ag ions. However, as it appeared that the biocidal effects of AgNPs might be partially due to Ag ion generation, further studies on this aspect of toxicity are required.
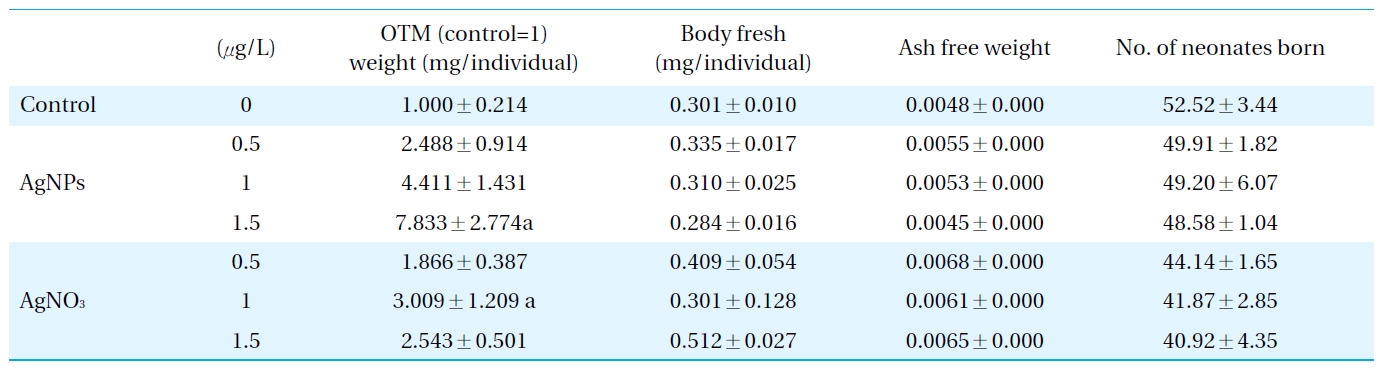
Growth, reproduction parameters investigated in Daphnia magna exposed to silver nanoparticles and AgNO3, and DNA damage (as OTM obtained by comet assay) measured in D. magna exposed to silver nanoparticles and AgNO3
In this study, the geno- and ecotoxicities of AgNPs on D. magnawere evaluated. The results suggested that AgNPs may havegenotoxic potential toward Daphnia, and AgNPs-induced DNAdamage might provoke higher-level consequences, which couldcomprise a contribution to the knowledge on the aquatic toxicityof AgNPs on aquatic ecosystems, for which little data areavailable. However, further studies on the mechanism behindAgNPs-induced DNA damage and mortality are needed to betterexplain the ecotoxicity of AgNPs in D. magna.
This research was supported by the International Research & Development Program of the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology(MEST) of Korea(Grant number: K20912000002-09B1300-00210) and the Korean Ministry of Environment through the Ecotechnopia 21 project