Harmful algal blooms, which usually present in stagnant water bodies, impact on water quality worldwide. Globally, the most commonly encountered cyanobacterial hepatotoxins are the cyclic peptide microcystins, which have been the cause of human and animal health hazards and even deaths.1) Microcystins potentially inhibit the function of eukaryotic protein serine/threonine phosphatases type 1 (PP1) and 2A (PP2A),2) which can lead to hepatocyte necrosis and hemorrhage at high levels.3) In addition to acute poisonings, exposure to low concentrations of microcystins in drinking water can cause chronic effects in mammals due to their potent tumor promoting activity.3) Epidemiological studies have shown that long term exposure to microcystins via drinking water supplies are associated with primary liver cancer. In 1996, for instance, the deaths of over 50 patients at a haemodialysis clinic in Brazil were attributed to microcystins, which were later identified in the clinic’s water supply.4) The increased rates of primary liver cancer in some areas of China have also been attributed to the contamination of drinking water with microcystins.3)
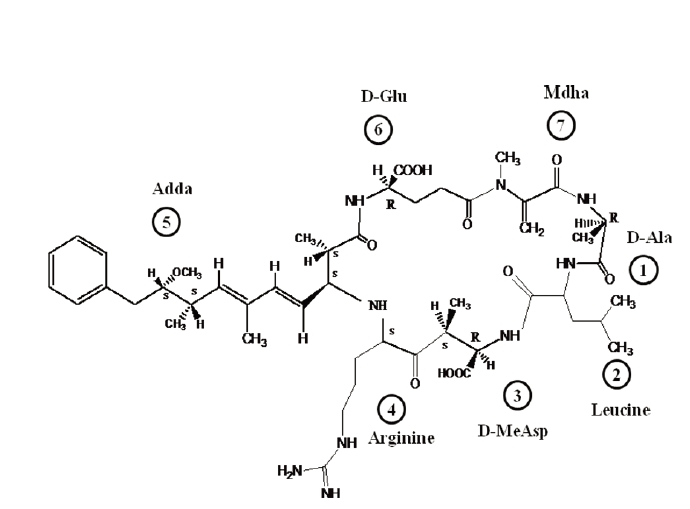
Of more than 80 microcystin variants identified to date, microcystin-leucine-arginine (MC-LR) has been the most extensively investigated due to its toxicity and frequent presence in cyanobacterial blooms in rivers and lakes all over the world.1,2) Microcystin-LR is a monocyclic heptapeptide hepatotoxin, which contains seven peptide-linked amino acids, with the molecular structure cyclo - (d-alanine [1] - leucine [2] - d-MeAsp [3] - arginine [4] - adda [5] - d-glutamate [6] - mdha [7]), in which d-MeAsp is d-erythro-β-methylaspartic acid, mdha N-methyldehydroalanine and adda (2S, 3S, 8S, 9S)-3-amino-9-methoxy-2, 6, 8-trimethyl-10-phenyldeca- 4E,6E-dienoic acid (Fig. 1).5) Leucine and arginine are protein amino acids, while the other five are non-protein amino acids and more or less constant between variant microcystins. MC-LR is an amphiphatic molecule. Hydrophilic functional groups, including carboxyl groups on glutamic acid and methylaspartic acid, as well as the amino group on arginine, while the Adda residue is hydrophobic.
Microcystins are intracellular secondary metabolites only produced by certain strains of cyanobacteria.7) These toxins are synthesized within the cells and released mainly to the surrounding water when the cells are lysed or become old and leaky.1,2) During the growth phase, microcystins are, for
most part, intracellular (fewer than 10 to 20% of total toxins are extracellular).8) It is the potential chronic toxicity from microcystins that led the World Health Organization (WHO) to establish a guideline of 1 μg/L as a maximum concentration of total MC-LR in drinking water.2) In addition, MC-LR was classified as a carcinogen according to the International Agency for Research on Cancer (IARC).9) Microcystin-LR was chosen as a representative microcystin in this study because it is the most toxic and abundant variant. Moreover, the commercial availability of MC-LR standard makes it easy to gain reproducible results.
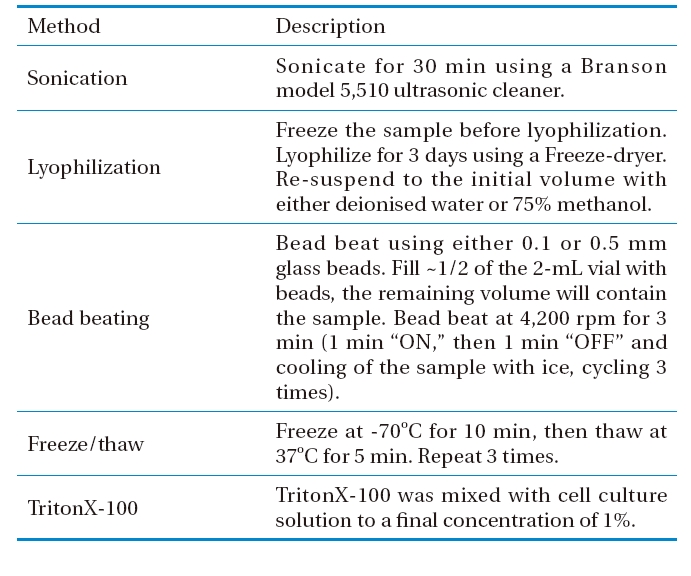
Prior to analysis of microcystin-LR, effective sample preparation steps are required.10,11) Ass the provisional guideline level of both intracellular and extracellular MC-LR in drinking water is as low as 1 μg/L, the outer membranes of cyanobacterial cells have to be disrupted. In the literature, several cell lysis methods that affect the extraction yield of microcystins have been used in the laboratory, including successive freeze-thaw cycles, autoclaving, sonication, heating, lyophilization, and the use of detergents, such as TritonX-100. However, there is no conclusive evidence regarding approach that performs best.12) For example, sonication is the most commonly employed method of extracting microcystins. However, this has been found to degrade commercial MC-LR standard solutions, and prolonged use of probe sonicators should be avoided as small samples might reach high temperatures and evaporate or degrade. Also, glass fiber filters, used for biomass concentration and drying, disintegrate and release fibers, which can interfere the analysis.13) A concentration step prior to analysis is also required, as extraction of cyanobacterial cells typically results in large volumes of solvent that contain relatively dilute amounts of MC-LR.12) Moreover, a concentration step is also required to remove the many impurities from the complex sample matrices in order to extend the liquid chromatographic column life.
In this study, the efficiency of various methods for lysing cyanobacterial cells to release intracellular toxins, and then for extracting microcystins from the cell lysates were evaluated. Isolation and concentration of MC-LR for analysis via high performance liquid chromatography coupled with electrospray ionization and a mass spectrometer (HPLC-ESI-MS), a widely accepted technique for determining MC-LR, were also investigated.
All chemicals and reagents were of either HPLC or analytical-reagent grade. The microcystin-LR standard was purchased from Alexis Biochemicals (San Diego, CA, USA). HPLC grade water, acetonitrile and methanol were purchased from Fisher Scientific Korea Ltd. (Seoul, Korea). Formic acid was purchased from Fluka Chemical Corp (Milwaukee, WI, USA). Deionised water was obtained by passing tap water through a Minipore system (Simpak Kit 1; Millipore, Paris, France) with the resistance >18.2 ㏁/cm. BG11 powder was obtained from Fluka BioChemika (Buchs, Switzerland).
2.2. Cultivation of Microcystis aeruginosa
2.3. Efficiency of Cell Lysis Methods
Different methods for disrupting M. aeruginosa cells were studied, including freeze/thaw, sonication, lyophilization, bead beating, and 1% TritonX-100 surfactant, as described in Table 1. The numbers of cyanobacterial cells, both before and after treatment, were measured by direct microscopy counting. Cells were injected into the counting chamber of an improved Neubauer hamocytometer (Marienfeld, Germany), with images captured by a LSM5 (Zeiss, Germany) inverted confocal laser scanning microscope (CLSM), equipped with a PC, using the LSM software (PASCAL) to control all the system components. Imaging was achieved using a C-Apochromat 40× objective. The efficiency of each cell lysis method was calculated via dividing the number of disrupted cells after treatment by the initial number of cells; this was then expressed as a percentage.
[Table 1.] Methods for Microcystis aeruginosa cell lysis
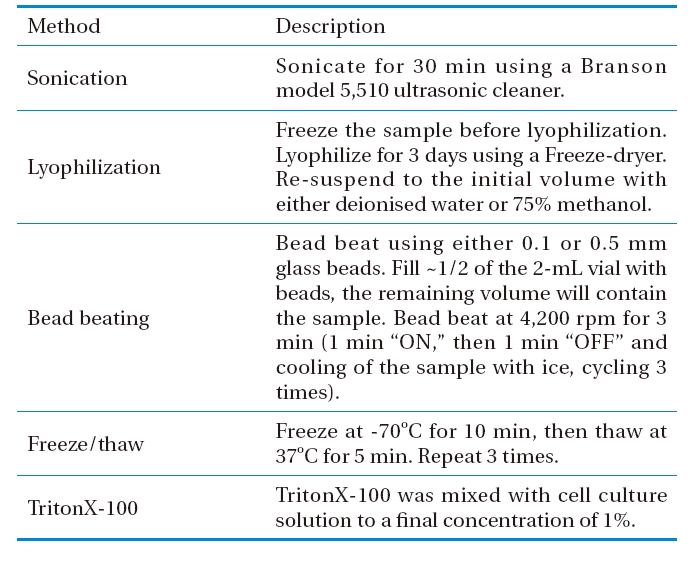
Methods for Microcystis aeruginosa cell lysis
E (%) = (Ci-Cf)/Ci × 100%
where, E(%) : Efficiency (%) of a cell lysis method
Ci : The initial concentration of M. aeruginosa cells (i.e. before cell lysis)
Cf : The final concentration of M. aeruginosa cells (i.e. after cell lysis)
2.4. Recovery Efficiency of MC-LR through a C18 Solid Phase Extraction Cartridge
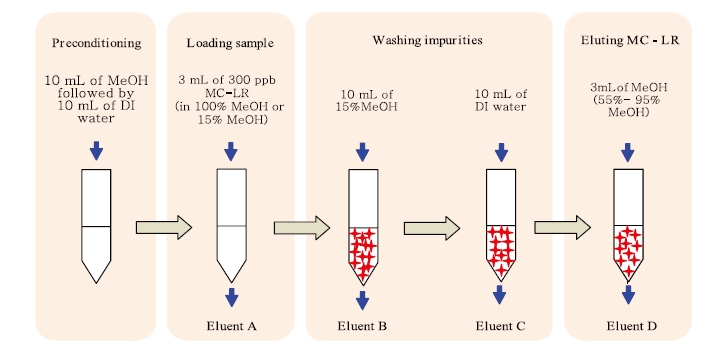
Microcystin-LR was concentrated on a C18 Sep-Pak/Vac 3 cc, 500 mg solid phase extraction (SPE) cartridge (Waters Corporation, Milford, MA, USA) using an extractor and vacuum pump (Waters Corporation) at a flow rate of 5 mL/min. The procedure can be briefly described as follows: 3 mL of 300 μg/L MC-LR, prepared in either absolute methanol or 15% methanol, was applied to a preconditioned SPE cartridge. The preconditioning step included washing with 10 mL of methanol, followed by 10 mL of deionised water. The cartridge was then washed with 10 mL of 15% methanol and 10 mL of deionised water to remove the undesired impurities. Finally, the target peptide was eluted using 3 mL of different percentages of methanol, ranging from 55 to 95% at 10% increments. All eluted fractions from loading, washing and eluting steps were collected and analyzed by HPLC-ESI-MS, which was performed using a Waters Alliance 2695 HPLC pump coupled to an electrospray (ESI) triple stage quadrupole Quattro micro (Waters Micromass; Waters Corporation) mass spectrometer (Fig. 2).
The HPLC system, consisting of a separation module (Waters 2695; Waters Corporation), an autosampler (Waters Alliance 2695; Waters Corporation) and a photodiode array detector (Waters 996; Waters Corporation), was controlled by MassLynx 4.1 software. The chromatographic separation was performed on a SunFire C18 column (2.1 mm i.d., 150 mm length, 3.5 μm pore size) using a mobile phase of acetonitrile and water containing 0.1% formic acid at a flow rate of 0.3 mL/min. The gradient conditions for a standard sample were: 0 min: 25% organic, 4 ? 5 min: 75% organic, 10 min: 25% organic. The volume of sample injected was 10 μL.
An electrospray (ESI) triple stage quadrupole Quattro micro (Waters Micromass; Waters Corporation) mass spectrophotometer was used to determine the molecular mass of MC-LR. Ions were formed in the positive ionization mode [M - H]+. The ion spray voltage was set at 3.5 kV, the source temperature at 150℃ and the desolvation temperature at 350℃.
3.1. Efficiency of Cell Lysis Methods
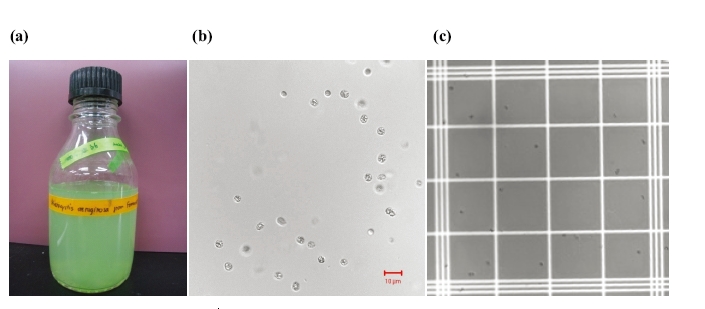
The cell lysis process was carried out to release intracellular materials, assisting the more efficient extraction of toxins of interest. Fig. 3(a) shows a visual image of the laboratory batch culture of
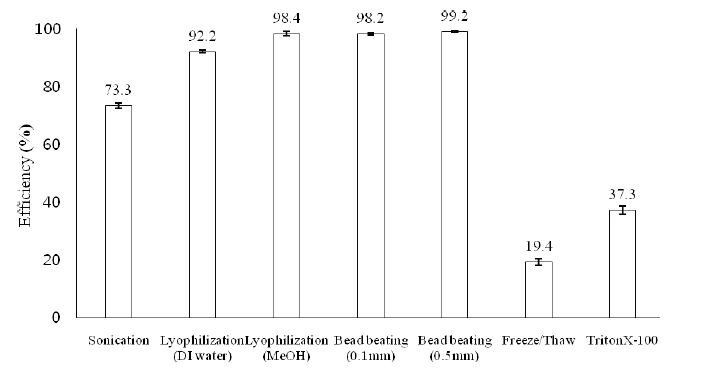
Before being lysed, the M. aeruginosa cell number was determined as 5.7±0.9×106 cells/mL via direct microscopic counting on the haemocytometer. After the treatments using various cell lysis methods, as described in section 2.3, the efficiencies of those methods were calculated to be 73.3±0.9% for sonication in a water bath, 92.2±0.5% for lyophilization followed by extraction with deionised water, 98.4±0.7% for lyophilization followed by extraction with 75% methanol, 98.2±0.3% for bead beating using 0.1 mm glass beads, 99.2±0.3% for bead beating using 0.5 mm glass beads, 19.4±1.2% for three sequential freeze/thaw cycles, and 37.3±1.4% for 1% TritonX- 100 surfactant, as depicted in Fig. 4.
The results obtained showed that lyophilization followed by the extraction of freeze- dried cyanobacterial mass with 75% methanol, the best solvent for microcystins extraction reported in literature, was sufficient to disrupt the
3.2. Recovery Efficiency of MC-LR through a C18 Solid Phase Extraction Cartridge
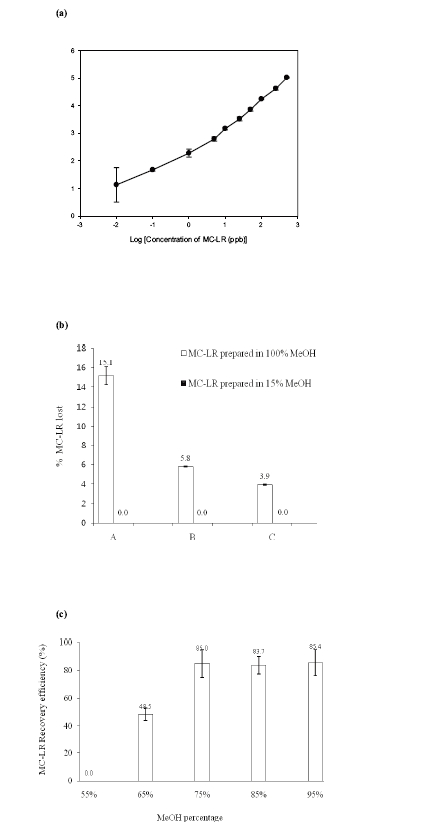
A calibration curve of the MC-LR precursor ion (m/z = 995.6) was plotted to evaluate the linearity of the HPLC-ESI-MS method (Fig. 5(a)). A lower limit of quantification of 1 ppb was obtained, with a correlation coefficient (r2) value higher than 99%. The assay proved to be linear and acceptable.
3.2.1. Loss in Loading and Washing Steps
An experiment was carried out with MC-LR variant to determining the potential losses during the loading and washing steps of the concentration process using the solid phase extraction method. Fig. 5(b) showed the losses of MC-LR during the loading and washing steps when the loaded MC-LR solutions contained different percentages of methanol. Applying MC-LR prepared in high percentage of methanol (100%) led to considerable losses during the loading (elutant A: 15.1±0.9%) and washing steps (elutant B: 5.8±0.0%, and C: 3.9±0.1%), approximately 24.8 ± 1.0% in total. Therefore, as the losses were under detection range in all steps, the MC-LR lost appeared to be insignificant when prepared in a low percentage of methanol (15%) prior to application to the C18 SPE cartridge. It was obvious that the percentage of methanol in the extract loaded onto the C18 SPE cartridge was a significant factor affecting the recovery efficiency of MC-LR. Therefore, reducing the percentage methanol from 75 to 15% was required after the solvent extraction step, which was achieved using a rotary evaporator.
3.2.2. Recovery Efficiency of Eluting Step
Fig. 5(c) showed the recovery efficiency of MC-LR when this toxin was eluted with different concentrations of methanol using a solid phase extraction method. It was found that employing methanol with percentages higher than 75% provided relatively similar recovery efficiencies for MC-LR, i.e. 85.0%±10.0% with 75% methanol, 83.7%±7.4% with 85% methanol, and 85.4%±9.8% with 95% methanol. A low concentration of methanol; 55%, was unable to elute MC-LR at a detectable concentration because MC-LR is relatively hydrophobic. Since 75% methanol offers the ability to extract analytes with a wide range of polarities, as reported in many previous publications, this percentage was chosen for further experiments to observe the recovery efficiency of MC-LR together with many other MC variants.
Of all the cell lysis methods evaluated, lyophilization followed by extraction with 75% methanol yielded the best result for releasing the intracellular toxin and extracting the target toxin from the cell lysates. The conventional C18 SPE method was appropriate for isolating and concentrating MC-LR containing only low concentrations of methanol, which was then eluted with methanol percentages higher than 75%. Therefore, after extracting MC-LR from the biomass, dilution of the extraction solvent methanol to a low concentration (approximately 15%) is recommended before loading onto the C18 SPE cartridge.