A powder form of aluminum (hydr)oxides is not suitable in wastewater treatment/filtration systems because of low hydraulic conductivity and large sludge production. In this study, aluminum (hydr)oxide-coated sand (AOCS) was used to remove phosphate from aqueous solution. The properties of AOCS were analyzed using a scanning electron microscopy (SEM) combined with an energy dispersive X- ray spectrometer (EDS) and an X-ray diffractometer (XRD). Kinetic batch, equilibrium batch, and closed-loop column experiments were performed to examine the adsorption of phosphate to AOCS. The XRD pattern indicated that the powder form of aluminum (hydr)oxides coated on AOCS was similar to a low crystalline boehmite. Kinetic batch experiments demonstrated that P adsorption to AOCS reached equilibrium after 24 h of reaction time. The kinetic sorption data were described well by the pseudo second-order kinetic sorption model, which determined the amount of P adsorbed at equilibrium (qe = 0.118 mg/g) and the pseudo second-order velocity constant (k = 0.0036 g/mg/h) at initial P concentration of 25 mg/L. The equilibrium batch data were fitted well to the Freundlich isotherm model, which quantified the distribution coefficient (KF = 0.083 L/g), and the Freundlich constant (1/n = 0.339). The closed-loop column experiments showed that the phosphate removal percent decreased from 89.1 to 41.9% with increasing initial pH from 4.82 to 9.53. The adsorption capacity determined from the closed-loop experiment was 0.239 mg/g at initial pH 7.0, which is about two times greater than that (qe = 0.118 mg/g) from the kinetic batch experiment at the same condition.
Pollution of water bodies by phosphorus, an essential macronutrient, is a wide-spread environmental problem, causing eutrophication in lakes and seas and posing a great threat to aquatic environments. In environmental systems, the interaction between phosphate and aluminum (hydr)oxides has attracted considerable attention in two respects. The first concerns the reaction of phosphate with aluminum (hydr)oxide minerals in soils, which controls its availability to plants and leaching into water bodies.1-3) The second is related to the removal of phosphate with aluminum (hydr)oxides. Through the application of positivelycharged aluminum (hydr)oxides as adsorbents, phosphate removal can be enhanced in tertiary wastewater treatment systems.4-6)
The adsorption of phosphate ions (monovalent: H2PO4 -, divalent: HPO4 2-) to aluminum (hydr)oxide surfaces can be described by ligand exchange mechanism.7,8) In the adsorption process, phosphate ions can replace hydroxyl ions (OH-) on the surfaces of aluminum (hydr)oxides, forming inner-sphere complexes including monodentate, bidentate, and binuclear complexes.4,9,10) Also, this mechanism can be referred to as Lewis acid-base interaction in which phosphate ions (Lewis base) are adsorbed to the surface sites (Lewis acid) of aluminum (hydr)oxides.10,11) Additionally, electrostatic (Coulombic) interaction can occur between positively- charged surfaces of aluminum (hydr)oxides and negatively-charged phosphate ions, forming outer-sphere complex.2,4,10,12)
The removal of phosphate with aluminum (hydr)oxides has been investigated by several researchers. These studies have examined the sorption capacity of aluminum hydroxide [Al(OH)3] for phosphate,13) effect of pH on adsorption of phosphate to bauxite, which has a major mineral of boehmite [γ-AlO(OH)],4) enhancement of phosphate adsorption capacity through acid and heat treatments,14) adsorption isotherms, rate, and selectivity of phosphate to aluminum oxide hydroxide,5) influence of humic substances on phosphate adsorption on aluminum hydroxide, 15) competitive adsorption between phosphate and natural organic matter on aluminum hydroxide,16) role of the surface acid-base properties of aluminum (hydr)oxides [pseudo-γ- AlO(OH) and α-Al2O3] in phosphate adsorption.17)
Because of low hydraulic conductivity and large sludge production, however, a powder form of aluminum (hydr)oxides is not suitable in wastewater treatment/filtration systems. Therefore, various granular forms of aluminum (hydr)oxide-coated media have been developed by several researchers including aluminum-loaded zeolite,18) aluminum- coated silica sand and olivine,19) granular aluminum oxide hydroxide,6) activated aluminum oxide,20) and aluminum oxide-coated quartz sand.12) The aluminum (hydr)oxide-coated media can be applied to adsorptive filtration systems for phosphate removal. Among these media, aluminum (hydr)oxide-coated sand (AOCS) is quite attractive for phosphate removal in the water filtration because sand is cheaper and more easily available than other filter media.21,22)
The objective of this study was to investigate the removal of phosphate from aqueous solution using aluminum (hydr)oxidecoated sand (AOCS). The properties of AOCS were analyzed using a scanning electron microscopy (SEM) combined with an energy dispersive X-ray spectrometer (EDS) and an X-ray diffractometer (XRD). Kinetic batch, equilibrium batch, and closedloop column experiments were performed to examine the adsorption of phosphate to AOCS.
2.1. Aluminum (Hydr)oxide-coated Sand
Quartz sand (Jumunjin Silica, Korea) was used to prepare AOCS. Mechanical sieving was conducted with US Standard Sieves (Fisher Scientific) Nos. 35 and 10. Sand fractions with a grain size of 0.5 2.0 mm and a mean diameter of 1.0 mm were used in the experiments. Before use, sand was washed twice using deionized water to remove impurities on the surface, and wet sand was autoclaved at 121℃ and 17.6 psi for 20 min, cooled to room temperature, and oven-dried at 105℃ for 1 2 days. For the preparation of AOCS, AlCl3?6H2O (4.9 g) was dissolved in deionized water (100 mL), and the solution pH was adjusted with 6 N NaOH to pH 7.0. The quartz sand (200 g) was added to the AlCl3?6H2O solution and then mixed in a rotary evaporator (90℃, 80 rpm, 20 min) to remove water in the suspension by heating (Hahnvapor, Hahnshin Scientific Co., Korea). The coated sand was dried at 150℃ for 6 h, washed with deionized water, and then dried again using the same conditions. The powder form of aluminum (hydr)oxides made in our laboratory was characterized by a powder X- ray diffractometry (XRD, D5005, Bruker, Germany) with a Cu K radiation. The scanning electron microscopy (SEM) and energy dispersive X-ray spectrometer (EDS) analysis were performed using a scanning electron microscope (JSM 5410LV, JEOL, Japan) to examine the presence of Al and P on the coated sand.
2.2. Kinetic and Equilibrium Batch Experiments
Phosphate adsorption to AOCS was determined by using kinetic and equilibrium batch experiments. Kinetic batch tests were performed at the initial phosphate concentrations of 25 and 100 mg/L with initial pH of 7.0 and NaNO3 of 0.02M. Five grams of AOCS were added to 30 mL phosphate (KH2PO4) solution in 50 mL polypropylene conical tubes. The concentration of NaNO3 was fixed at 0.02M as background electrolyte. The solution pH was adjusted to pH 7 with 0.1M NaOH and/or 0.1M HCl. The tubes were shaken at 30±0.5℃ and 140 rpm using shaking incubator (SHAK116, Daihan Scientific, Korea). The sample was taken at 1, 3, 6, 9, 12, 24, and 48 h after reaction and filtered through 0.45㎛ membrane filter. Phosphate was analyzed by the ascorbic acid method.23) Phosphate concentration was measured at a wavelength of 880 nm using a UV vis spectrophotometer (Helios, Thermo, USA). Equilibrium batch tests were conducted with initial phosphate concentrations ranging from 10 to 200 mg/L. The same procedure used for the kinetic test was followed in the experiments with the sampling time of 24 h after reaction. All the experiments were performed in triplicate.
2.3. Closed-Loop Column Experiments
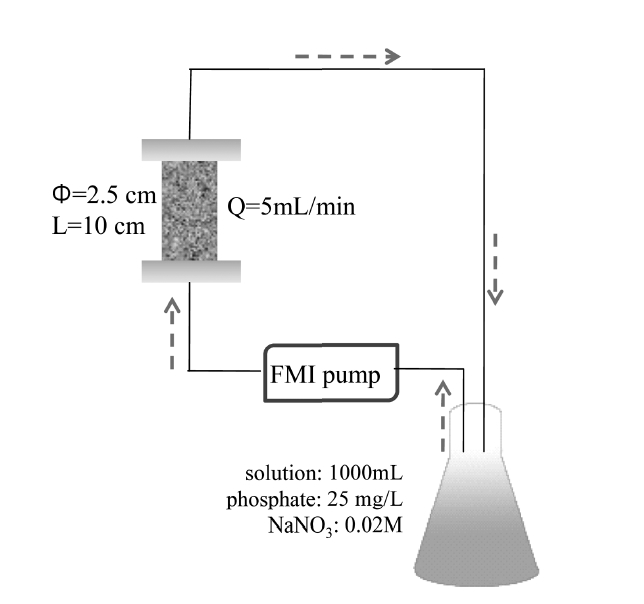
Closed-loop column experiments (Figure 1) were performed using a Plexiglas column (diameter: 2.5 cm; height: 10 cm) packed with AOCS (mass of medium: 75.58±1.24 g). A column was packed for each experiment by the tap-fill method to attain a bulk density of 1.565±0.008 g/cm3 and a porosity of 0.410± 0.003. A phosphate solution (25 mg/L, 1000 mL) was prepared with 0.02M NaNO3 as background electrolyte. The solution pH was adjusted to a desired value with 0.1M NaOH and/or 0.1M HCl. The column was connected to a FMI pump (QG400, Fasco, USA) operating at a rate of 5.0 mL/min. The experiment was performed by circulating the phosphate solution for 24 h. After completing the experiment, samples were collected and analyzed for phosphate concentration and pH. Phosphate concentration was determined as mentioned above. The pH was measured with a pH probe (9107BN, Orion, USA).
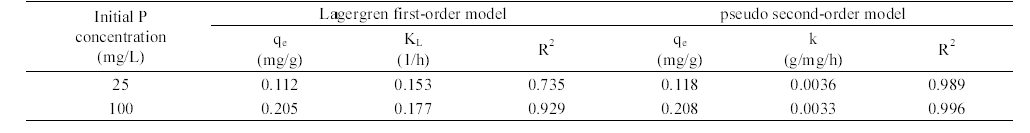
The adsorption kinetic data were analyzed by the following the Lagergren first-order and pseudo second-order models:24,25)
where
where
3.1. Characteristics of Aluminum (Hydr)oxide-coated Sand
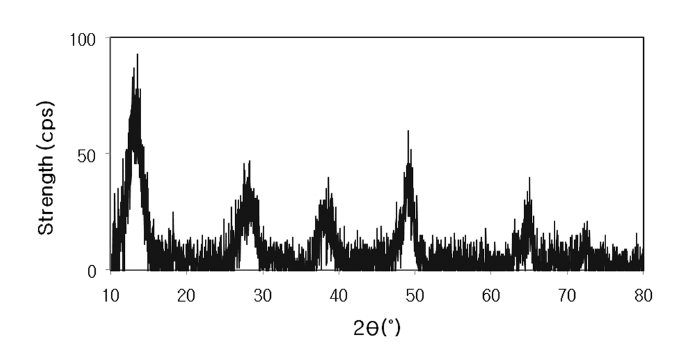
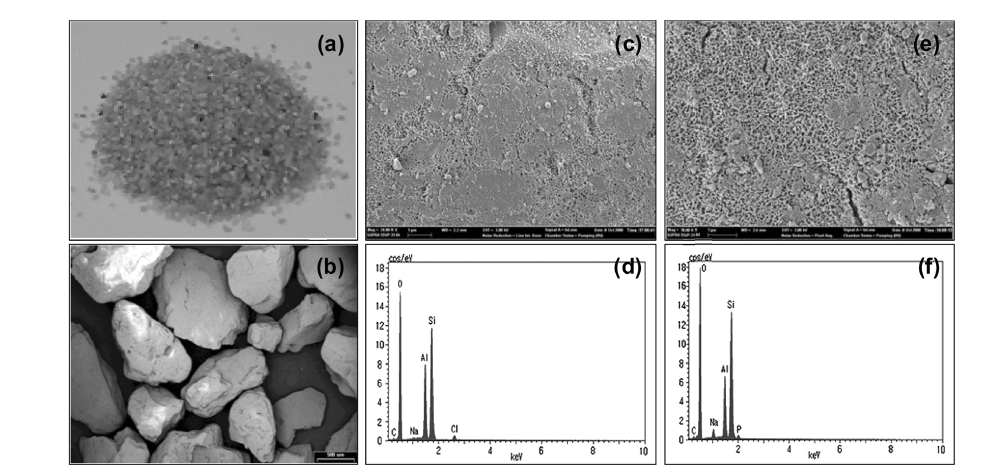
The XRD pattern for the powder form of aluminum (hydr) oxide is shown in Figure 2, which is similar to that of a low crystalline boehmite.5,11) The surface characteristics and chemical composition of AOCS was presented in Figure 3. The photo and SEM images showed the color, size, and shape of AOCS granules (Figure 3(a) and 3(b)). The SEM images of AOCS surfaces before and after phosphate adsorption were given in Figure 3(c) and 3(e), respectively, indicating that aluminum (hydr)oxide particles partly covered the surfaces of quartz sand. In addition, the EDS pattern after phosphate adsorption (Figure 3(f)) was similar to that before adsorption (Figure 3(d)), except for the phosphate peak. The major constituents of AOCS were Si and Al.
3.2. Phosphate Adsorption to Aluminum (Hydr)oxide-coated Sand
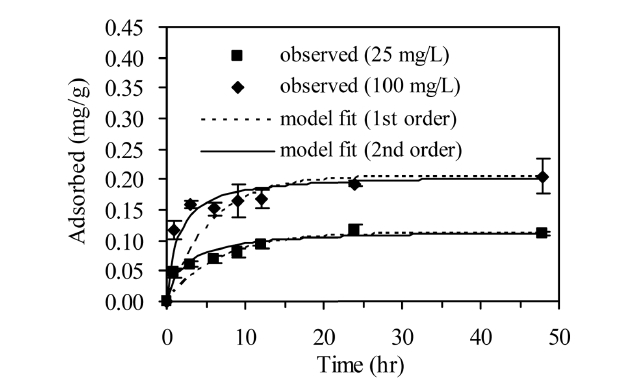
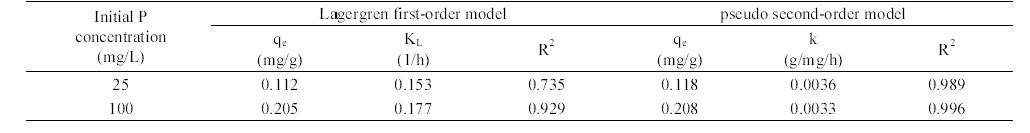
The adsorption kinetics of phosphate in AOCS is shown in Figure 4. The adsorption reached equilibrium after 24 h of reaction time. Model parameters for Lagergren first- order and pseudo second-order models obtained from the kinetic experiments are presented in Table 1. In the first-order model, the value of
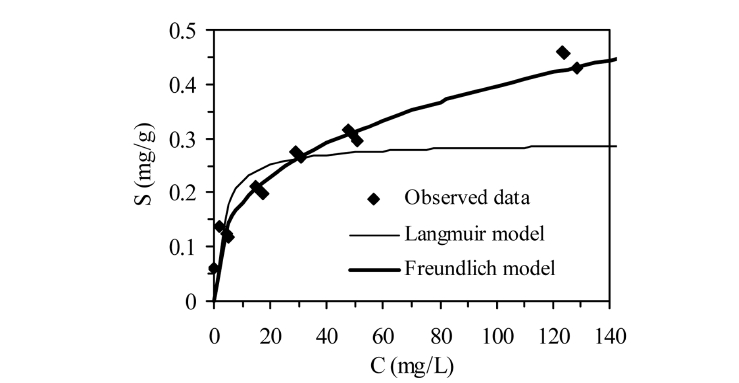
The equilibrium adsorption isotherms of phosphate on AOCS are presented in Figure 5. In the Freundlich model, the distribution coefficient (
isotherm model is used to describe the multilayer adsorption at adsorption sites.
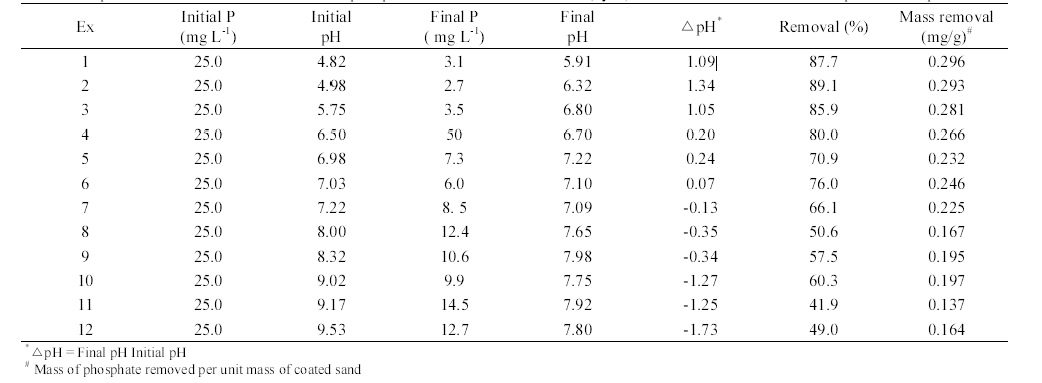
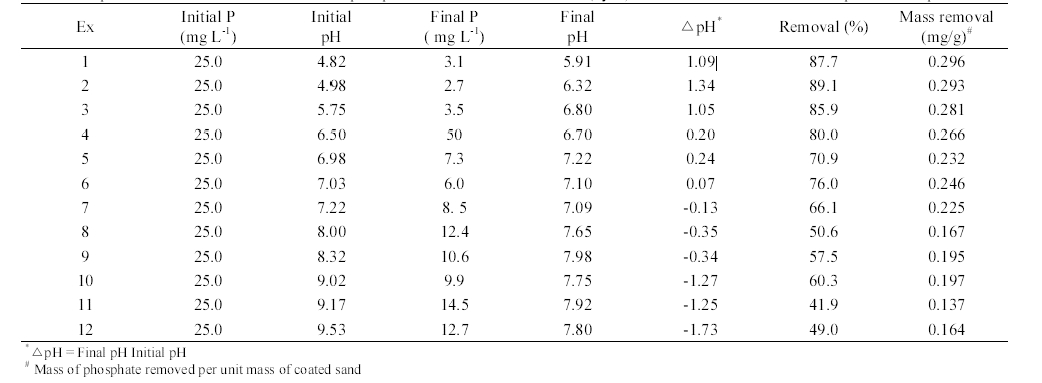
The phosphate removal in closed-loop column experiments is summarized in Table 2. The adsorption capacity of AOCS determined from the closed-loop experiments ranged from 0.296 to 0.137 mg/g, depending on the solution pH. At initial pH 7.0, the adsorption capacity was 0.239 mg/g (0.232 mg/g at pH 6.98, 0.246 mg/g at pH 7.03), which is about two times greater than that (qe = 0.118 mg/g) obtained from the kinetic batch experiment (pH 7.0, temperature 30℃, 0.02 M NaNO3) at the initial phosphate concentration of 25 mg/L. This discrepancy can be attributed to the differences in experimental type, medium-tosolution ratio, and among others. Note that the medium- to-solution ratio in kinetic batch experiments was 1:6 (medium of 5 g: solution of 30 mL) while it was 3.8:1 (bulk density of 1.565 g/cm3: porosity of 0.410) in closed-loop column experiments.
Model parameters for the Lagergren first-order model and pseudo second-order model obtained from the kinetic experiments
Experimental conditions and results for phosphate removal with aluminum (hydr)oxide-coated sand in closed-loop column experiments
3.3. Phosphate Removal and Solution pH
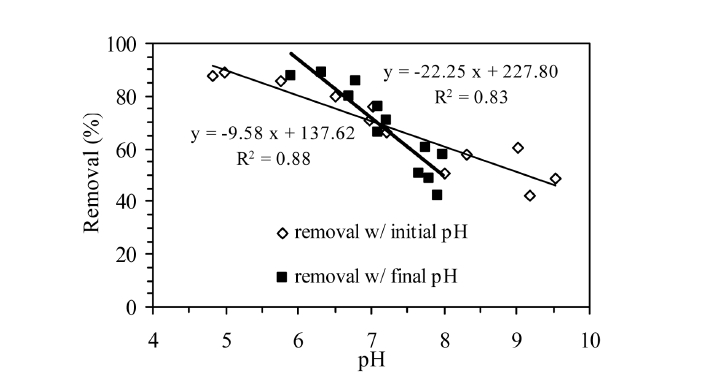
The phosphate removal percent in AOCS decreased with increasing solution pH (Figure 6). This decreasing tendency can be attributed to the charge modification of adsorption sites and the competition between phosphate ions and hydroxyl ions. As solution pH increases, the surface sites on aluminum (hydr)oxides become less positively-charged and further change to the negatively- charged above pHpzc (point of zero charge, 9.7).26) Furthermore, the concentration of hydroxyl ions increases with increasing pH, and thus the competition between phosphate ions and hydroxyl ions to the adsorption sites of aluminum (hydr) oxides is enhanced.18) Our result is consistent with the result of Tanada et al.5) who have shown that the amount of phosphate adsorbed to aluminum oxide hydroxide decreased with increasing initial pH from 4.0 to 8.5. Another batch experiment2) has shown that the adsorption density of orthophosphate (6 mM) to γ-Al2O3 decreased with increasing pH from 4.0 to 10.0. Other experiment3) has also demonstrated that phosphate content in solution after reaction with boehmite and γ-Al2O3 decreased with increasing pH from 3.0 to 11.0. The similar results were also found from other experiments.4,18)
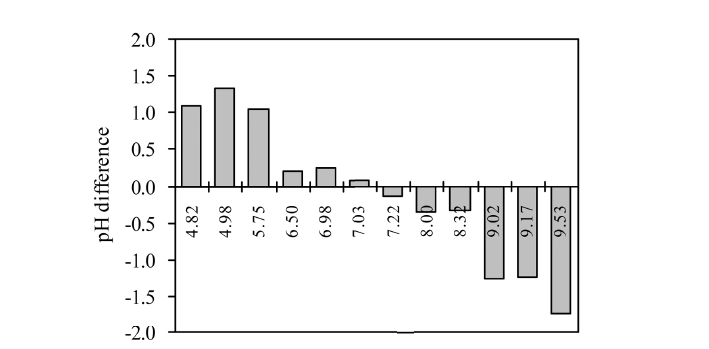
The pH differences (final pHs - initial pHs) determined from the closed-loop column experiments are presented in Figure 7. In the experiments 1-6 (Table 2), the pH differences between the initial and the final were positive and tended to decrease with increasing pH. Meanwhile, the pH differences between the initial and the final were negative and tended to decrease with decreasing pH in the experiments 7-2 (Table 2). It is well known
that ligand exchange is the main mechanism in the adsorption of phosphate to aluminum hydr(oxide) surfaces. Hydroxyl ions are released into the solution during the experiment, resulting in the increase of solution pH. In our experiments with a sufficient reaction time, phosphate ions can play a buffering role in the solution through protonation/deprotonation between H2PO4 - and HPO4 2-, tending to neutralize the solution pH. Between pH 4.0 and 7.0, the solution pHs increased due to the buffering role of phosphate ions in addition to the release of hydroxyl ions, while they decreased between pH 7.0 and 10.0 due to the role of phosphate ions against the release of hydroxyl ions. Our result is not consistent with the report of Tanada et al.5) who have shown that the final solution pHs were larger than the initial in all batch experiments for the phosphate adsorption to aluminum oxide hydroxide. Another experiment4) also demonstrated that all the solution pHs increased after the phosphate adsorption reaction in bauxite. This discrepancy may be ascribed to the different experimental conditions between their studies and ours including reaction time and solution volume, among others. In our experiments, reaction time was 24 h with solution volume of 1000 mL while it was 2 h with 100 mL in their batch experiments.4) Note that no information was provided from Tanada et al.5) regarding their reaction time and solution volume.
The adsorption of phosphate to AOCS was investigated in this study using kinetic batch, equilibrium batch, and closed-loop column experiments. The XRD pattern indicated that the powder form of aluminum (hydr)oxide coated on AOCS was similar to a low crystalline boehmite. Kinetic batch experiments showed that P adsorption to AOCS reached equilibrium after 24 h of reaction time. The kinetic sorption data were described well by the pseudo second-order kinetic sorption model while the equilibrium batch data were fitted well to the Freundlich isotherm model. The closed- loop column experiments showed that the phosphate removal percent decreased with increasing solution pH. This tendency can be attributed to the charge modification of adsorption sites and the competition between phosphate ions and hydroxyl ions. The adsorption capacity determined from the closed-loop experiment was about two times greater than that from the kinetic batch experiment at the same condition. This discrepancy can be ascribed to the differences in experimental type and medium-to- solution ratio.