Recovery of heavy metals from wastewaters and industrial wastes has become a very important environmental issue. Nickel has many useful applications in our life and equally harmful if discharged in appreciable quantities into natural water resources.1) Ni2+ is present in the effluents of silver refineries, electroplating, zinc base casting and storage battery industries. The acceptable limit of Ni2+ in drinking water is 0.01 mg/L and 2.0 mg/L as industrial wastewater discharge. At higher concentrations, Ni2+ can cause cancer of the lungs, nose and bone. Dermatitis- Ni itch which may occur as a result of contact with coins and costume jewelries is one of the most frequent effects of exposure to Ni2+. Ni carbonyl [Ni(CO)4] has been estimated as lethal in humans at atmospheric exposures of 30 ppm for 30 min.3) Acute poisoning of Ni2+ causes headache, dizziness, nausea and vomiting, chest pain, tightness of the chest, dry cough and shortness of breath, rapid respiration, cyanosis and extreme weakness.1,2) Hence, it is essential to remove Ni2+ from industrial wastewaters before it pollutes natural water sources. Conventional methods for removal of Ni2+ from wastewaters include chemical precipitation, ion exchange, adsorption unto activated carbon, filtration, chemical reduction, and electrodeposition.4) Due to operational demerits and high cost of heavy metal treatment, some new technologies have been tried in recent times with less expensive adsorbents such as rice hull,5) sphagnum peat6) and
Sorption characteristics of Ni2+ onto montmorillonite and evaluation of the clay’s potentialities as sorbent material for removal of Ni2+ from aqueous solution is the focus of this study. From batch adsorption studies, effects of pH, clay dosage, and concentration on Ni2+ adsorption unto montmorillonite were investigated. Equilibrium studies relating to kinetics, adsorption isotherms and thermodynamics were as well experimentally conducted.
Analytical grade of montmorillonite was purchased from Aldrich Chemicals. Clay fraction passed through a 150 μm sieve was used as received, unwashed powder. Its surface area as determined by the EGME method8) was found to be 699 m2/g and from an extraction method with ammonium acetate, a CEC of 89 meq/100g was obtained.
Stock solution of 1000 mg/L of Ni2+ was prepared by dissolving 4.960 g of ultra pure Ni(NO3)2. 6H2O in a double distilled water, acidified with nitric acid to prevent hydrolysis. All the solutions were made with double distilled water.
Batch adsorption studies were conducted. Adsorption kinetics were carried out using 50 mL of metal ion solution containing the desired concentration (50-300 mg/L) at a pH of 5.5 with 1 g of adsorbent in 100 mL conical flasks agitated at 200 rpm inside a rotary shaker (25±1℃). Samples were separated by fast filtration and analyzed by Flame atomic absorption spectrometer. Studies on adsorption kinetics were carried out using different initial Ni2+ concentrations with 1g of adsorbent dosage in 50 mL solution. 0.1 N of HCl and 0.1 N of NaOH were used to adjust the pH. The effect of hydrogen ion concentration was examined from solutions at pH ranging from 2.3 to 9.2, Ni2+ removal was studied in the range of 2.5-9.0.
Fig. 1 summarizes the sorption of Ni2+ onto montmorillonite particles at various pH values. The maximum removal was achieved at pH values around 7-9 due to the nature of the chemical interactions of the metal ion with the montmorillonite surface. At pH above 9, Ni2+ precipitates. Montmorillonite surface contains several different active sites4) and metal ion removal depends on these active sites as well as on the nature of the metal ions in the solution. Greater number of negatively charged groups on montmorillonite favours electrostatic interactions between cationic species, and this negative charge may be responsible for metal binding. However, as the pH is lowered, hydrogen ions compete with metal ions for the sorption sites in the sorbent; the overall surface charge on the particles becomes positive and hinds the binding of positively charged metal ions (Ni2+). Hydrogen ion concentration affects not only active sites dissociation, but also the metal speciation. Hydrolysis products of metal cations can also be investigated as metal cations at around pH 5 would be expected to interact with the negatively charged binding sites of montmorillonite.9-12)
Fig. 2 shows the adsorption of Ni2+ as a function of clay dosage. Increase in montmorillonite dosage increased the adsorption of Ni2+, this may be attributed to availability of more func-
tional groups as well as active sites for adsorption. Large numberof sites were available for each fixed concentration of sorbatehence the increase in extent of adsorption.
3.3. Adsorption Kinetics (effect of contact time and initial metal ion concentration)
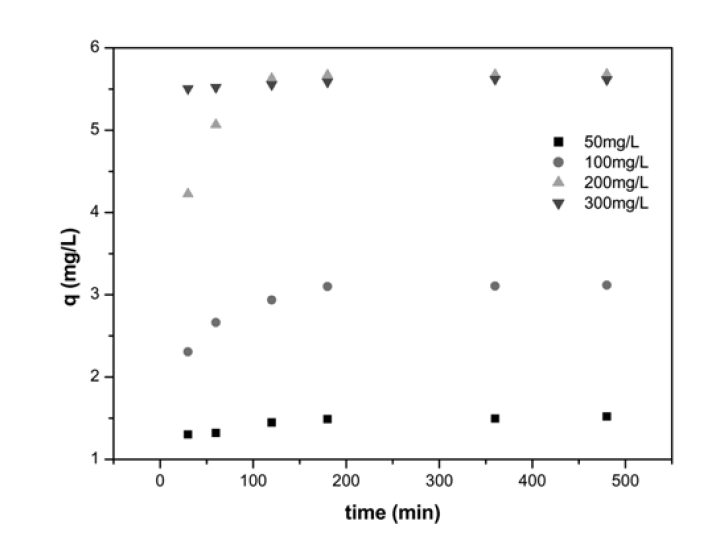
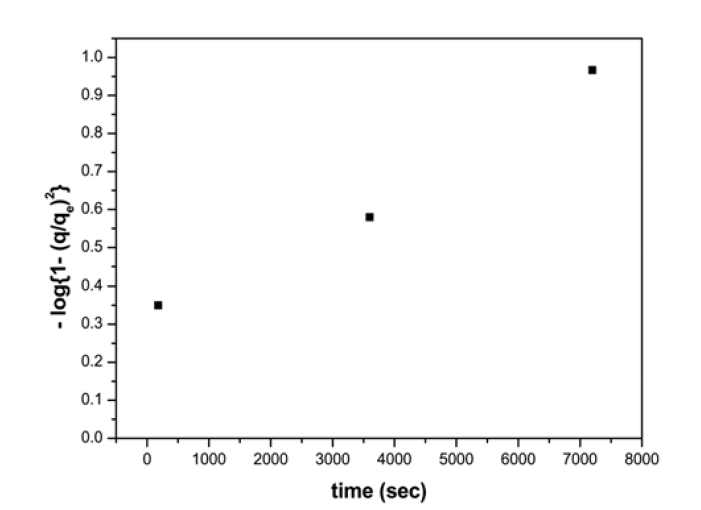
Solute uptake rate, governing residence time of sorption reactions is one of the important characteristics in defining sorption efficiency. The effect of agitation time and initial Ni2+ concentration on adsorption is presented in Fig. 3 with the equilibrium time selected as the agitation time for the batch experiments. The progressive increase in adsorption and consequently the attainment of equilibrium adsorption may be due to limited mass transfer of the adsorbate molecules from the bulk liquid to the external surface of montmorillonite initially, and subsequently by slower internal mass transfer within the montmorillonite particles. Similar trends were also observed for polyvinyl alcohol adsorption onto montmorillonite.13) Sorption kinetic was analyzed employing two models, first, using Lagergren equation, which allows the estimation of the adsorption rate
where,
The pseudo-second-order model was equally applied using14):
where
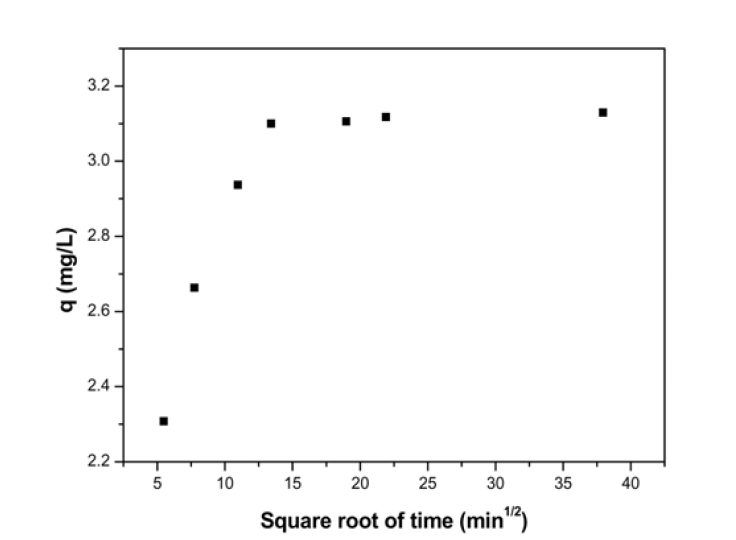
It is always important to predict the rate-limiting step in an adsorption process in order to understand the mechanism associated with the phenomena. For a solid liquid sorption process, the solute transfer may be characterized by an external mass transfer, intraparticle diffusion or by both transport phenomena. Three types of mechanisms are involved in adsorption process15): the film diffusion, which involves the movement of adsorbate molecules from the bulk of the solution towards the external surface of the adsorbent; the particle diffusion, where the adsorbate molecules move and being sorbed in the interior of the adsorbent particles; retention on active sites through sorption, complexation or intraparticle precipitation. Of the three steps, the third step is assumed to be very fast and hence considered negligible. Therefore for design purposes, it is required to clearly distinguish between film diffusion and particle diffusion in order to establish identify the slowest step in the adsorption process. Intraparticle diffusion is characterized by the relationship between specific sorption (
where
Fig. 4 shows a non-linear distribution of points, with two separate portions of a curve and a linear plot for the sorption process and as such indicating the existence of intraparticle dif
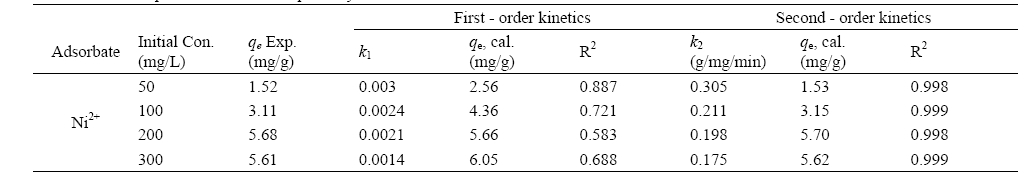
[Table 1] Kinetic parameters for Ni2+ uptake by montmorillonite at different concentrations
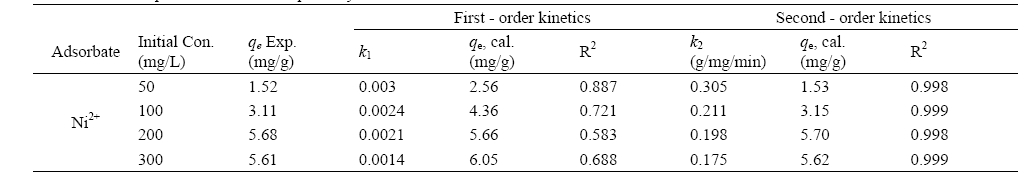
Kinetic parameters for Ni2+ uptake by montmorillonite at different concentrations
fusion process. According to the intraparticle diffusion model, if a plot of the amount of sorbate adsorbed per unit weight of sorbent,
The Langmuir model was originally developed to represent chemisorption on a set of well-defined localized adsorption sites independent of surface coverage; having the same adsorption energy; and with no interaction between adsorbed molecules. This model, also called as the ideal localized monolayer model, is valid for monolayer sorption onto a surface with a finite number of identical sites19) and is given by Equation 4
where
From the data of 1/
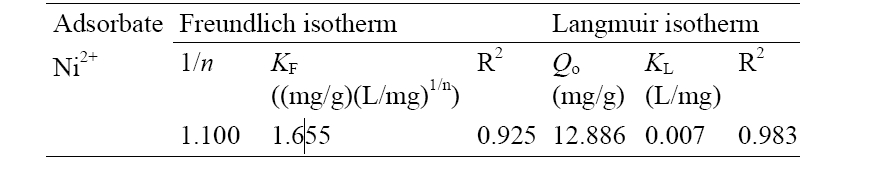
The Freundlich adsorption isotherm usually fits the experimental data over a wide range of concentrations. This empirical expression encompasses the surface heterogeneity and exponential distribution of the active sites and their energies. The widely used empirical Freundlich equation based on sorption on a heterogeneous surface19) is given by Equation 6
where
The values of
[Table 2] Freundlich and Langmuir parameters for Ni2+ sorption
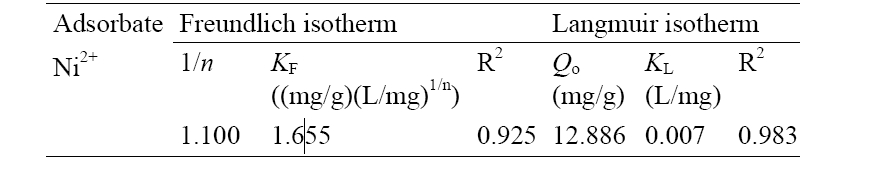
Freundlich and Langmuir parameters for Ni2+ sorption
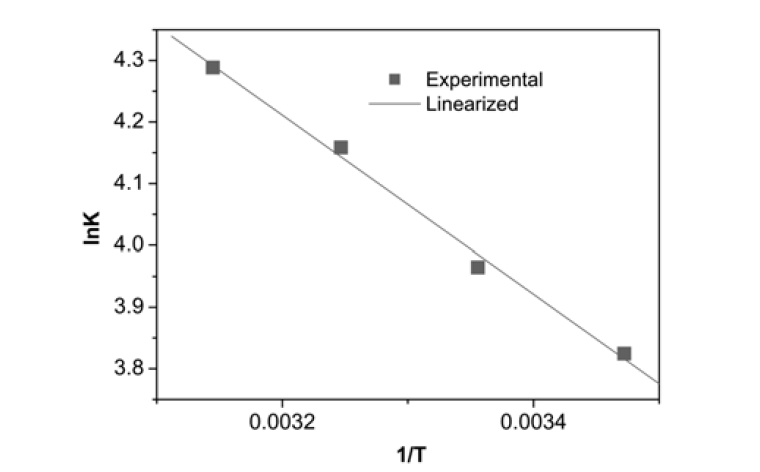
The thermodynamic parameters for the adsorption process, Δ
The values of log
where ΔS° and ΔH° are entropy (kJ/mol K) and enthalpy (kJ/mol) change of adsorption, respectively,
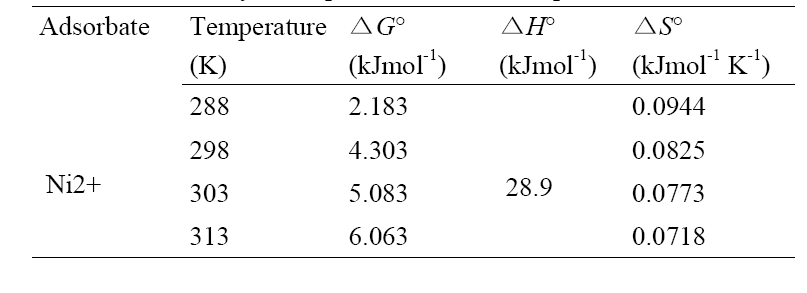
The plot shown in Fig. 6 for Ni2+ was linear at the range of temperature investigated. The calculated thermodynamic parameters such as ΔH°, ΔS° and ΔG° are given in Table 3. The positive values of ΔG° indicate that the sorption of Ni2+ onto montmorillonite is not a spontaneous process. The change in enthalpy (Δ
[Table 3] Thermodynamic parameters for Ni2+ uptake
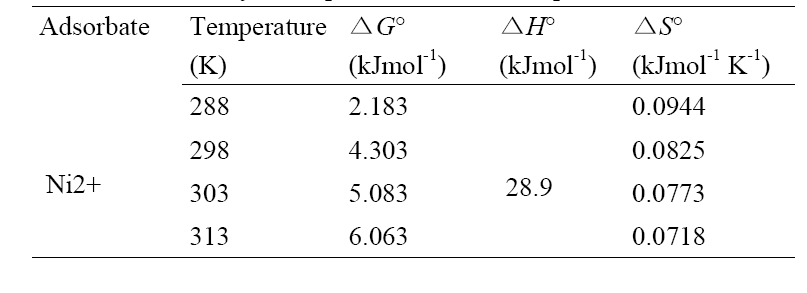
Thermodynamic parameters for Ni2+ uptake
temperature favors adsorbate transport within the particles of the adsorbent. The positive low values of ΔS° indicate low randomness at the solid/solution interface during the uptake of Ni2+ by montmorillonite.
Adsorption characteristics of Ni2+ onto montmorillonite and the potential use of montmorillonite as sorbent material for Ni2+ have been studied. Nickel as representative of heavy metals is chosen for this study as it is present in effluents of many industries. Ni2+ was sorbed due to strong interactions with the active sites of the sorbent. Montmorillonite was able to remove Ni2+ from aqueous solutions and the equilibrium experimental data fitted well to Langmuir than the Freundlich isotherm model. The pseudo first- order and pseudo-second-order models were employed to fit the adsorption kinetics and as such the adsorption of Ni2+ onto montmorillonite followed the pseudo-secondorder kinetics. Diffusion of Ni2+ inside the clay particle was confirmed as the rate-controlling step and more important for Ni2+ adsorption rate than the external mass transfer. Thermodynamic parameters (Δ
Ce : equilibrium concentration of solution (mg/L)
ΔG° : change in Gibb’s free energy of adsorption (kJ/mol)
ΔH° : change in enthalpy of adsorption (kJ/mol)
ΔS° : change in entropy of adsorption (kJ/mol K)
KF : Freundlich isotherm constant related to adsorption capacity ((mg/g)(L/mg)1/n)
KL : intensity of adsorption (L/mg)
m : clay mass (g)
n : Freundlich isotherm constant related to adsorption intensity
q : amount of heavy metal adsorbed at time t (mg/g)
qe : amount of adsorbed heavy metal per unit clay mass (mg/g)
Qo : maximum adsorption capacity (mg/g)
R2 : correlation coefficient
R : gas constant (Jmol/K)
V : volume of solution (L)
KN : Van’t Hoff equilibrium constant
f : uptake percentage of adsorbate at equilibrium.
T : absolute temperature (K)
t : time (min)
![Change in residual concentration of Ni2+ solution according to pH (amount of adsorbent: 1g; 25℃; 200 rpm; metal ion [Ni] = 100 mg/L).](http://oak.go.kr/repository/journal/10036/E1HGBK_2009_v14n1_26_f001.jpg)
![Variation of the adsorption of Ni2+ onto montmorillonite according to sorbent dosage (25℃; 200 rpm; pH∼5.5; metal ion [Ni] = 100 mg/L).](http://oak.go.kr/repository/journal/10036/E1HGBK_2009_v14n1_26_f002.jpg)