The use of plant and plant products in treatment of diseases is as old as mankind. Traditional and herbal medicines being natural are still preferred to contemporary synthetic medicines by a major section of the world. According to the World Health Organization (WHO), 80% of the world population relies on herbal medicines, for their primary health care needs (WHO, 2007). The major merits of traditional or herbal medicine seem to be their perceived efficacy, low incidences of serious adverse effects and comparatively low cost.
Accumulation of toxic industrial effluents in the soil, air and water is continuously increasing due to rapid urbanization and extensive pollution of the environment. Among these toxic substances, presence of heavy metals (atomic weights 63.5 - 200.6 g/mol and a specific gravity greater than 5 g/cm3) which are ubiquitous in nature, cause serious harmful effects on living organisms especially humans (Nies, 1999).
Plants are sensitive to environmental conditions and they accumulate these heavy metals in their harvestable parts (via root uptake, foliar adsorption and deposition of specific elements in leaves) and intensity of this uptake process can change the overall elemental composition of the plant (Olajire and Ayodele, 2003). Some of the heavy metals namely arsenic, lead, cadmium and mercury are not essential for plants and these are insidiously toxic. The possibility that toxic heavy metals can be translocated to humans and animals through the use of herbs grown in polluted areas is a major concern for traditional and herbal medicine (Yap et al., 2010). Consumption of herbal products from the medicinal plants, grown in polluted sites can cause serious consequences on human health. For getting desirable therapeutic benefits, quality of these raw herbs must be ensured in terms of heavy metal contamination. Due to this reason, WHO advocates that herbs and herbal products should not be used without qualitative and quantitative analysis of their heavy metals contents (WHO, 2007).
The medicinal plants-related trade in India has been estimated at Rs 5000 chores per annum with an annual growth rate of 7 - 15% (Joshi et al., 2004), may face serious consequences if heavy metal accumulation beyond permissible limits is detected. Therefore, assessment of these hazardous metals in raw herbal material and finished products must be undertaken compulsorily. Heavy metal analysis of raw herbs should be prioritized so that the contamination cannot accumulate up to the finished products. This may be regarded as raw material quality assurance strategy.
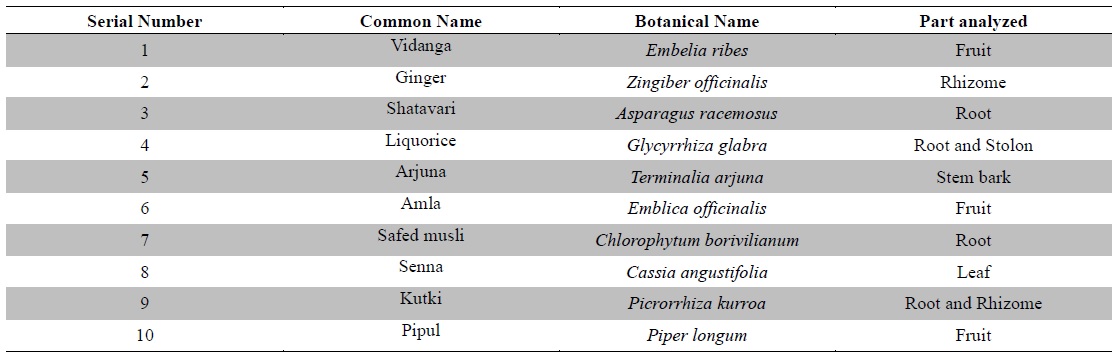
Hence, there is a relevant necessity for assessment of these heavy metals in medicinal plants to ascertain the level of heavy metal contaminants in herbal raw materials. The present study was designed to quantitatively analyze the levels of six potentially toxic heavy metals namely arsenic (As), lead (Pb), cadmium (Cd), mercury (Hg), chromium (Cr) and nickel (Ni) in ten raw medicinal herbs (Table 1). The medicinal plants were selected on the basis of their established-medicinal properties, significant importance in Ayurveda, the traditional system of Indian medicine, along with their frequent utilization in traditional as well as contemporary pharmaceutical, dietary and cosmetics preparations worldwide.
[Table 1.] Plant materials used in the study
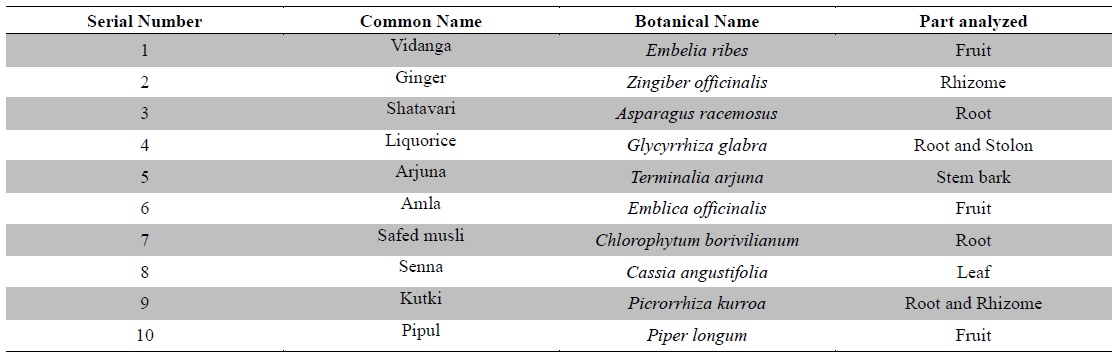
Plant materials used in the study
Ten raw medicinal herbs (Table 1) were procured from the medicinal plant supplier namely M/s. S. N. Das & Co., Burrabazar, Kolkata 70001, West Bengal, India. The plant materials were authenticated at the Central National Herbarium, Botanical Survey of India, Shibpur, Howrah, West Bengal, India. The plant materials were shade dried and homogenized and ground mechanically into coarse powders and subjected to analysis.
Supra pure nitric acid was form J. T. Baker and analytical grade hydrogen peroxide was from Merck. Standard stock solutions (NIST traceable) of As, Pb, Cd, Hg, Cr, Ni were from Merck. All the solutions and dilutions were prepared by using Milli-Q water (Millipore, Elix).
The sample of each plant material was prepared by microwave assisted wet digestion. Briefly, about 0.5 g of powdered plant material was mixed with 5 ml of nitric acid (68%) and 1 ml of hydrogen peroxide (30%) solution in a clean dry Teflon digestion tube and subjected to microwave assisted digestion in a microwave digester (CEM Corp., USA). After digestion the digest was filtered and quantitatively transferred into 25 ml volumetric flask and made up to volume with Milli-Q water. Further dilutions were made if necessary.
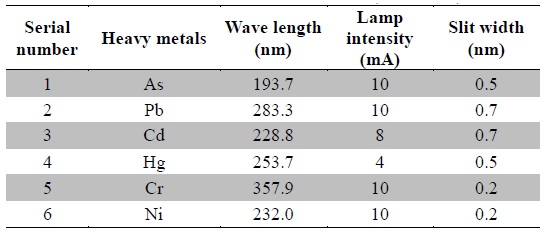
The standard stock solutions (1000 ppm) were diluted to obtain working standard solutions ranging from 1 ppb to 15 ppb and stored at 4℃. The calibration curves were plotted between measured absorbance and concentration. The prepared samples were immediately analyzed using atomic absorption spectrophotometer (AAS, Agilent 280FS AA) equipped with graphite tube atomizer (GTA 120). The instrument was operated in GTA mode, the argon gas flow was 3 L/min and the temperature parameters were followed as recommended by the instrument manufacturer. Optimized operating parameters of various heavy metals are listed in Table 2. All analyses were run in batches, which included standards (for calibration curves), reagent blanks and plant samples. The heavy metal concentrations were expressed in parts per million (ppm) with respect to the dry weight of the plant materials. All the samples were analyzed in duplicates and the result averaged.
[Table 2.] Operating parameters for the instrument (AAS-GTA)
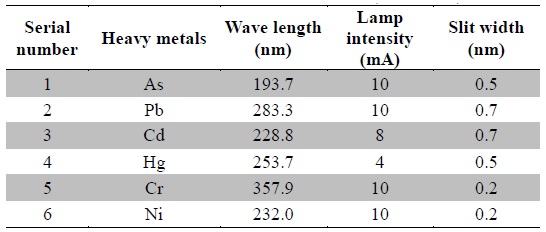
Operating parameters for the instrument (AAS-GTA)
Atmosphere and soil are continuously being polluted with chemicals and heavy metals due to dynamic development of industries and motorization along with extensive use of pesticides and fertilizers. In turn, these pollutants and heavy metals are getting accumulated in the plants growing in the polluted areas, which subsequently enter the human food chain via plant parts and extracts or preparations thereof. The environmental impact of these metals as well as their adverse health effects has been a source of major concern worldwide.
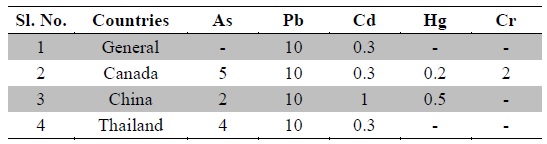
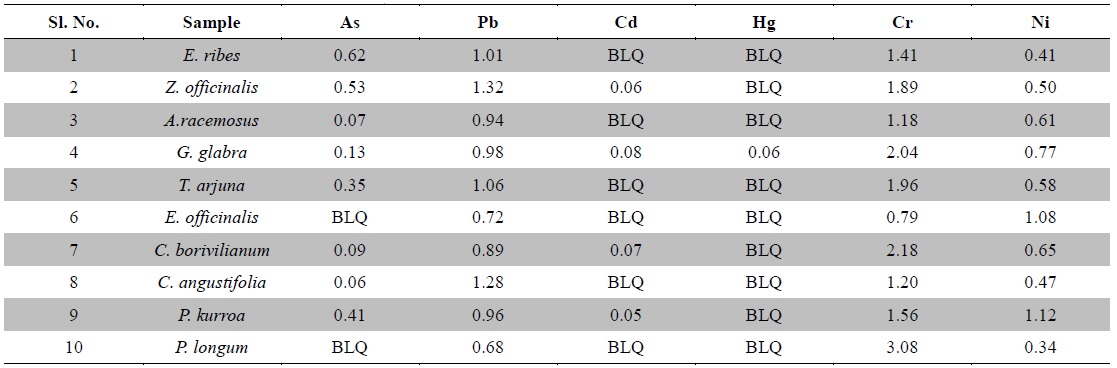
Nowadays, Indian medicinal plants have been regarded as a potential source of heavy metal toxicity to both man and animals (Dwivedi and Dey, 2002). The most common heavy metals implicated in human toxicity include arsenic, lead, cadmium and mercury although nickel and chromium may also cause toxicity. Except nickel, WHO prescribes limits of all five toxic heavy metals in raw medicinal herbs (WHO, 2007) (Table 3). In India, likewise WHO, the Ayurvedic Pharmacopoeia of India recommends that medicinal plants, which form the raw materials for most herbal remedies, should be checked for the presence of the first four above said heavy metals especially and prescribes limits for them (Anonymous, 2009). However, majority of Indian people, living in areas where these plants grow, harvest them locally for their own medicinal use without checking for heavy metal accumulation. Most of the Indian Ayurvedic and herbal companies procure the raw herbs from commercial suppliers and use in formulations without checking them for heavy metals. In the present study, ten important medicinal raw herbs recognized in Ayurveda were subjected to quantitative heavy metal analysis by AAS. The results of analysis of six heavy metals in ten raw medicinal herbs are summarized in Table 4.
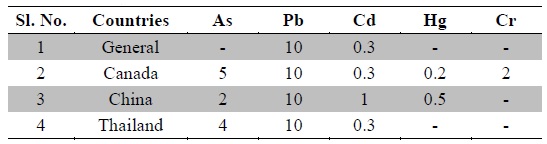
Permissible limits for toxic heavy metals in raw medicinal herbs (ppm) as per WHO (WHO, 2007)
[Table 4.] Heavy metal contents in medicinal herbs (ppm)
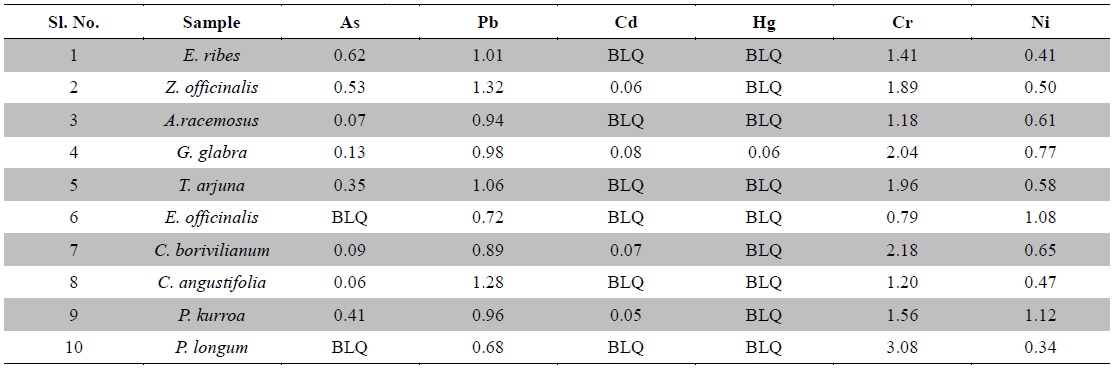
Heavy metal contents in medicinal herbs (ppm)
Arsenic is a toxic non-essential element. Its contamination is caused from geological sources leaching into aquifers, contaminating water and may also occur from mining, pesticide application and other industrial processes. It is present as a contaminant in many traditional remedies. Groundwater arsenic toxicity in is a serious public health problem affecting many millions of people in Ganges delta incorporating Eastern India (West Bengal state) and Bangladesh (Bhattacharya et al., 2014). Trivalent arsenic is more toxic to mammals than its pentavalent form. Arsenic exerts its toxicity by inactivating up to 200 enzymes, especially those involved in cellular energy pathways, DNA synthesis and repair. Acute arsenic poisoning is associated initially with nausea, vomiting, abdominal pain, severe diarrhea, encephalopathy and peripheral neuropathy. Chronic toxicity results multisystem diseases including carcinogenesis affecting almost all organs (Ratnaike, 2003; Bhattacharya et al., 2014). Maximum permissible limit for arsenic in raw herbs is 3.0 ppm as per the Ayurvedic Pharmacopoeia of India (Anonymous, 2009). Here, arsenic was not detected in
Lead is the most frequently occurring and stable heavy metal in nature. It is highly hazardous for plants, animals and micro-organisms. Continuous application of fertilizers, fuel combustion and sewage sludge are the major reasons leading to escalation in lead pollution. It is a non-essential element that can be introduced to human by inhalation, ingestion or cutaneous absorption. It is a serious cumulative body poison. Levels of lead beyond the permissible limits or long term use of these contaminated plants could lead to toxicity characterized by colic, anemia, chronic nephritis, headache, convulsions, brain damage and central nervous system disorders (Klaassen, 2001; Tong, 2000). The Ayurvedic Pharmacopoeia of India recommends the maximum permissible limit of lead is 10.0 ppm in raw herbs (Anonymous, 2009). In the present study, lead was detected in all plant samples and the values were around 1 ppm which was well below the prescribed limit by the pharmacopoeia and WHO.
Cadmium is a non-essential trace element with uncertain direct functions in both plants and humans and responsible for several cases of poisoning through food. Recently, it is gaining more attention due to wide occurrence in water, soil, milk, dietary and herbal medicinal products (Singh et al., 2014). Small quantities of cadmium cause adverse changes in the arteries of human kidney leading to kidney failure. It accumulates in human body, replaces zinc biochemically and causes hypertension, liver and kidney damage. Cadmium poisoning causes a disease called Itai-itai characterized by softening of bones, anemia, renal failure and ultimately death (Nordberg, 1999). The maximum allowable limit for cadmium in raw herbs is 0.3 ppm as per Ayurvedic Pharmacopoeia of India (Anonymous, 2009). In the present analysis, cadmium was not detected in
Mercury can cause adverse effects on the renal and nervous systems and can cross the placental barrier, with potential toxic effects on the fetus (Risher and WHO, 2003)). Mercury exposure for the general population occurs mainly from consumption of fish, as methyl mercury and possibly from dental amalgam fillings (Bhat and Moy, 1997). Levels of mercury beyond the allowable limits have been associated with infertility, inhibition of endogenous antioxidant enzymes and brain damage. As per Ayurvedic Pharmacopoeia of India, the maximum permissible limit of mercury in raw herbs is 1.0 ppm (Anonymous, 2009). In the present study, mercury was not detected in the plant samples tested except
Chromium contamination is caused by tanneries, paper, paint and steel industries; and sewage sludge applications along with alloys in motor vehicles. Chromium is essential in carbohydrate metabolism. It also functions in protein and cholesterol biosynthesis. It is an important element required for the maintenance of normal glucose metabolism. The function of chromium is directly related to the function of insulin, which plays a very important role in diabetes mellitus. Chromium is found in the pancreas, which produces insulin (Ano and Ubochi, 2008; Zetić et al., 2001). The toxic effects of chromium intake are skin rash, nose irritations, bleeds, stomach upset, kidney and liver damage and lung cancer. Chromium deficiency is characterized by disturbance in glucose lipids and protein metabolism (Rai et al., 2005; Shanker et al., 2005). The permissible limit for chromium in raw herbal materials is 2.0 ppm in Canada as per WHO (Table 3). In the present investigation, except
The most common ailment arising from Nickel is an allergic dermatitis known as nickel itch, which usually occurs when skin is moist, further more Nickel has been identified as a suspected carcinogen and adversely affects lungs and nasal cavities. Although Nickel is required in minute quantity for body as it is mostly present in the pancreas and hence plays an important role in the production of insulin. Its deficiency results in the disorder of liver (Pendias and Pendias, 1992). Nickel was recognized as an allergen of the year in 2008 by the American Contact Dermatitis Society and its minimal risk level was set to 0.2 μg/m3 for inhalation during 15 - 364 days, however, no limit has been set for food stuffs and herbs (Bhat et al., 2010). In the present experiment, less than 1.5 ppm nickel content was found in all the test samples. WHO does not define any permissible limit for nickel neither in raw herbs nor in herbal products.
The Ayurvedic Pharmacopoeia of India however, does not prescribe any limit for chromium and nickel neither in raw herbs nor in finished medicinal products.
Form the present investigation, it can be concluded that except the chromium content of
The authors have no conflicting financial interests.