Pharmacopuncture is a new form of acupuncture treatment combining acupuncture and herbal medicine. With acupuncture based on the meridian theory and herbal medicine based on Qi and flavor theory, pharmacopuncture utilizes both meridian and Qi/flavor theory. Pharmacopuncture treatment requires differential diagnosis by a traditional Oriental medicine practitioner to select a matching herbal extract, and the injections are given at several acupoints to attain benefits of both acupuncture and herbal medicine [1]. Thus, a single procedure, can achieve both the effects of acupuncture and herbal medicine. As the pharmacopuncture does not pass through the digestive system, it works fast and has effects that oral administration does not have [2]. For this reason, pharmacopuncture for herbal medicine has been widely used.
The constituents of the Eun-Bi San pharmacopuncture are
Studies on the toxicities of the components Eun-Bi San pharmacopuncture have been published. Seo and Roh conducted a philological study on the poisoning and side effects of
Studying the acute and the subacute toxicities through Good Laboratory Practice (GLP) regulations is the current research trend for single-dose toxicity testing of extracts. All the experiments for this study were conducted at Biotoxtech.
The Eun-Bi San pharmacopuncture was made in a clean room at the KPI (K-GMP). After the mixing process with sterile distilled water, the pH was controlled to between 7.0 and 7.5. Then, NaCl was added to make a 0.9% isotonic solution by using sterilized equipment. The completed extract was stored in a refrigerator (2.1 ─ 5.5°C), until it was used. The date of manufacture on this extract was 2013-4-21, and its valid date was 2013-10-21.
In this study, six-week-old Sprague-Dawley (SD) rats reared by ORIENTBIO were used. The reason SD rats were chosen is that they have been widely used in safety tests in the field of medicine, so the results can be easily compared with many other data-bases. The weights of the male rats ranged from 177.6 to 193.2 g, and those of female rats ranged from 150.3 to 176.8 g at the time of injection. Upon import, for all animals, a visual inspection was conducted; all animals were weighed using a CP3202S system (Sartorius, Germany). During 7 days of acclimatization, the general symptoms of the rats were observed once a day. The weights of the rats were recorded on the last day of acclimatization. No abnormalities were found.
The temperature of the breeding environment was 21.1 ─ 22.7°C, the humidity was 44.3% ─ 56.6%, and the illumination was 150 ─ 300 Lux. Feedstuff (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and UV filtered water were provided.
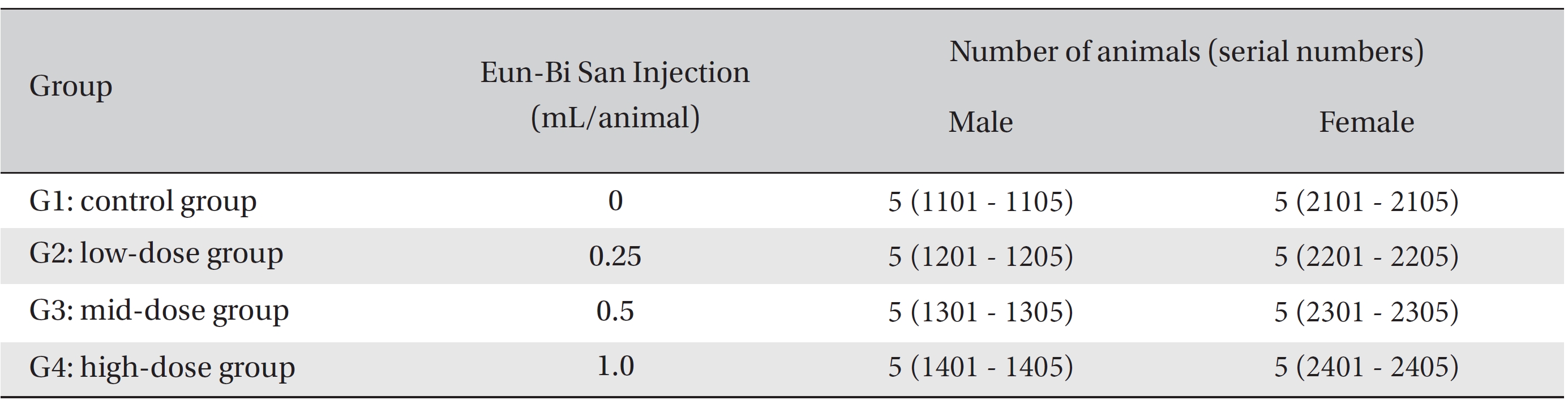
After 7 days of acclimatization, animals were selected and grouped by using the criteria of their weights being close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were randomly distributed into 4 groups (5 male and 5 female mice per group), as shown in Table 1.
Because the usual dose for Eun-Bi San pharmacopuncture is 1.0 mL per treatment in clinical applications, 1.0 mL of Eun-Bi San pharmacopuncture was injected into each male and female rat in a pilot test. In this study, 1.0 mL/ animal was set as a high dose, and 0.5 mL/animal and 0.25 mL/animal were set as the mid dose and the low doses, respectively. In the control group, 1.0 mL of normal saline solution was administered. Disposable syringes were used to inject a single dose, 0.25 and 0.5 mL/animal, of Eun-Bi San pharmacopuncture into the left thigh muscle of the rats in the low dose and the mid dose groups, respectively, 0.5 mL of Eun-Bi San pharmacopuncture was injected into each thigh muscles of the rats in the high dose group, for a total of 1.0 mL/animal. This study was performed under the approval of the Institutional Animal Ethics Committee of Biotoxtech Co., Ltd.
The general symptoms were observed once a day from the 1st day to 14th day after treatment. On the administration day (day 0), the general symptoms (kinds of side effects, revealing time, recovery time, etc.), as well as mortality, were examined at 30 minutes and at 1, 2, 4, and 6 hours after injection. The weights were measured immediately before treatment and at 3, 7 and 14 days after treatment.
After fasting for more than 18 hours before autopsy, the rats were anesthetized by using isoflurane. Blood samples were taken from the abdominal aorta on the day of autopsy (15 days after injection). A blood sample of about 1 mL was analyzed by using an automatic hematology analyzer (ADVIA 120, SIEMEMS, Germany). A blood sample of about 2 mL was centrifuged for the blood coagulation test (3,000 rpm, 10 minutes). The results were measured by using an Automated Coagulation Analyzer (Coapresta 2000, SEKISUI, Japan). The blood obtained from the abdominal aorta was analyzed in the blood biochemical test. The results were measured by using an Automatic Analyzer (7180, HITACHI, Japan) and Electrolyte Analyzer (AVL9181, Roche, Germany). Finally, for all animals, the organs and tissues of the body were visually inspected and microscopically observed.
The weights and the hematologic examination and blood chemical test results obtained from the experiments were analyzed by using statistical analysis system (SAS, version 9.3, SAS Institute Inc., U.S.A.). A Bartlett test was conducted to assess the homogeneity of the variance and the significance. The one way analysis of variance (ANOVA) test was carried out when the homogeneity of the variance was recognized, and the Kruskal-Wallis test was conducted post-hoc.
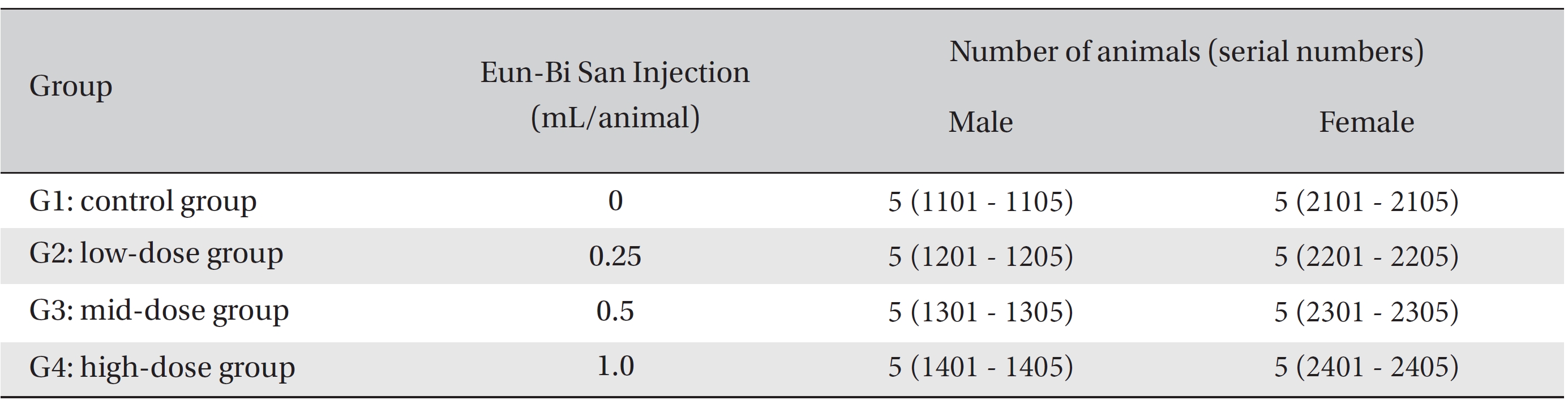
Groups of animals
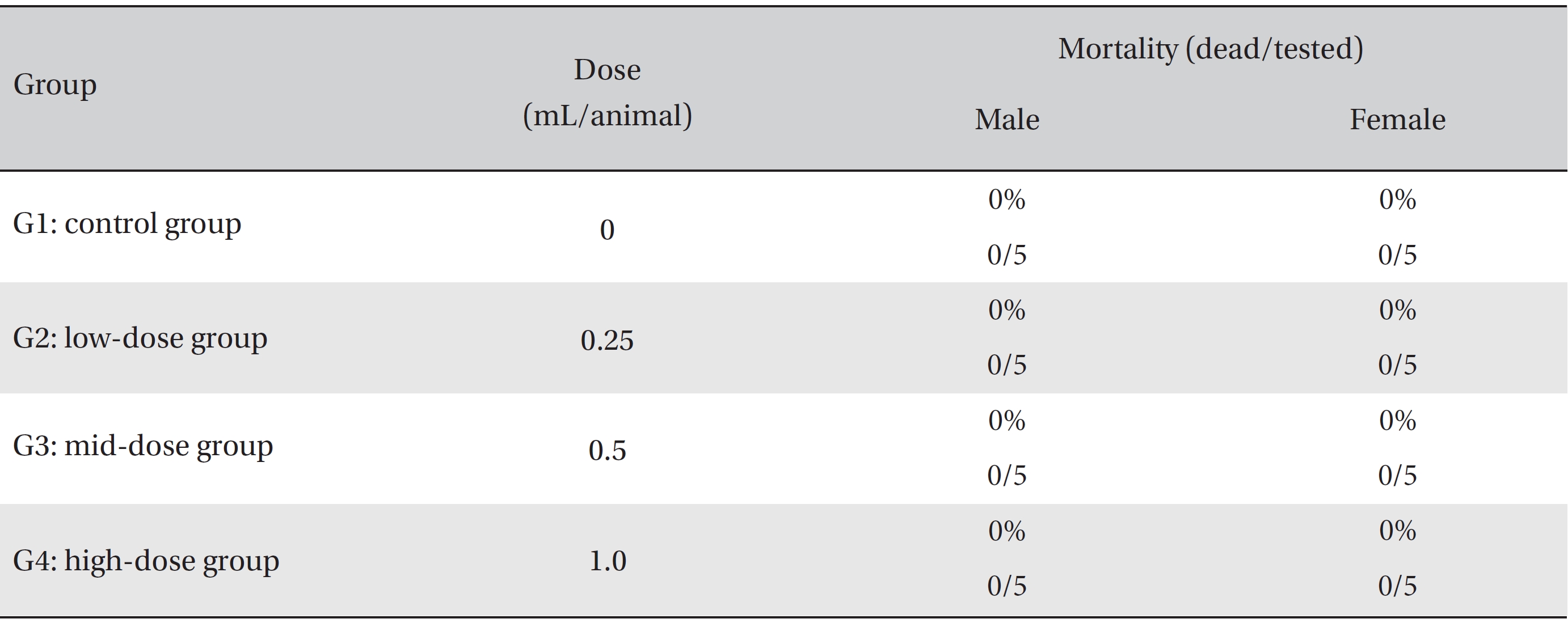
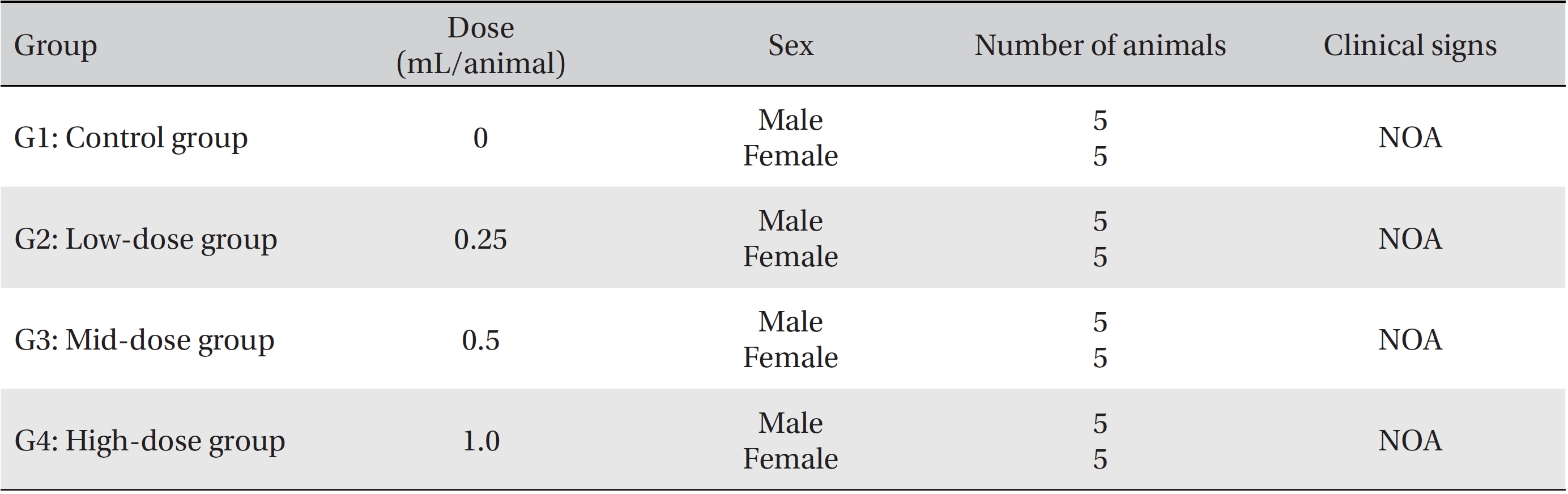
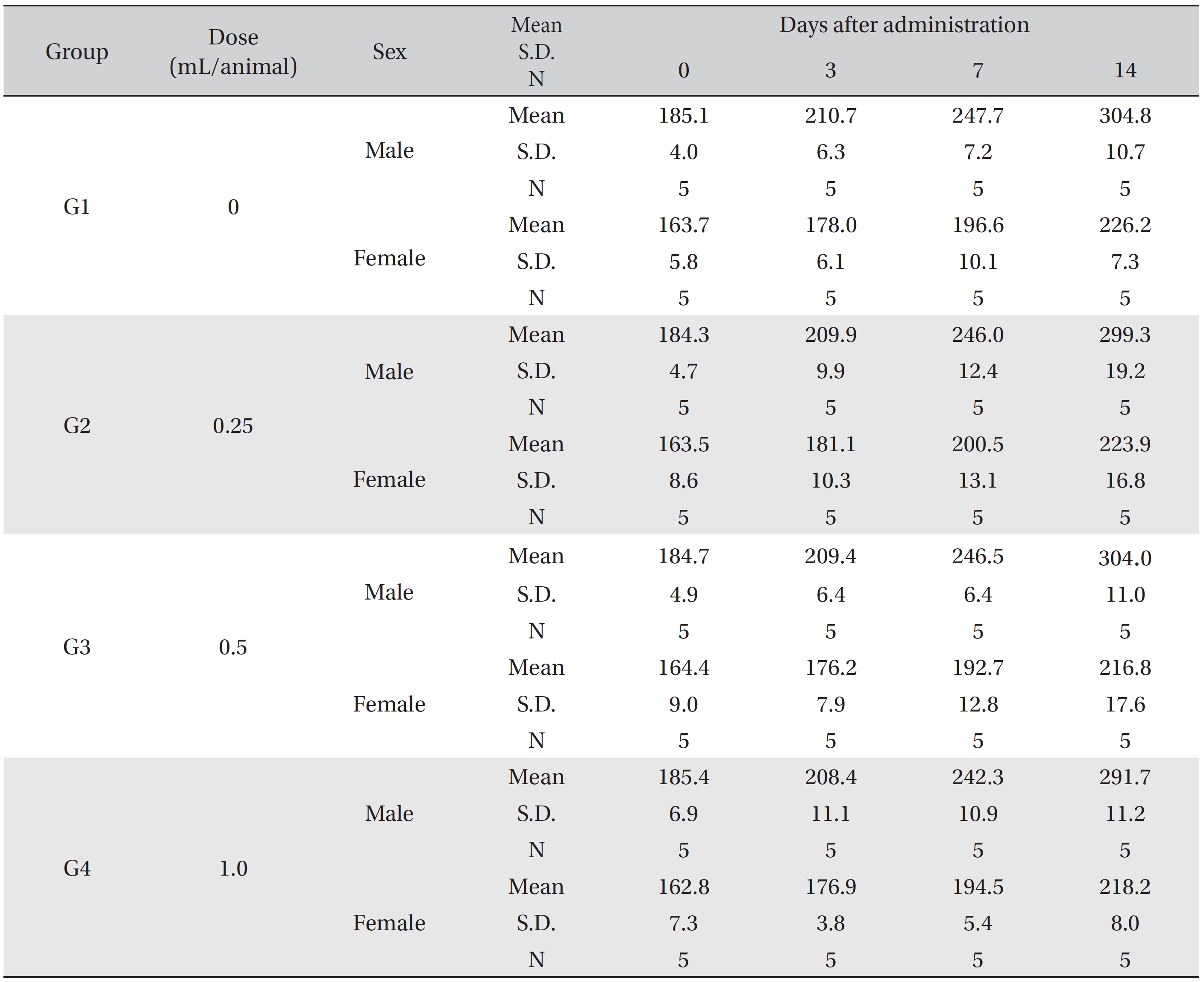
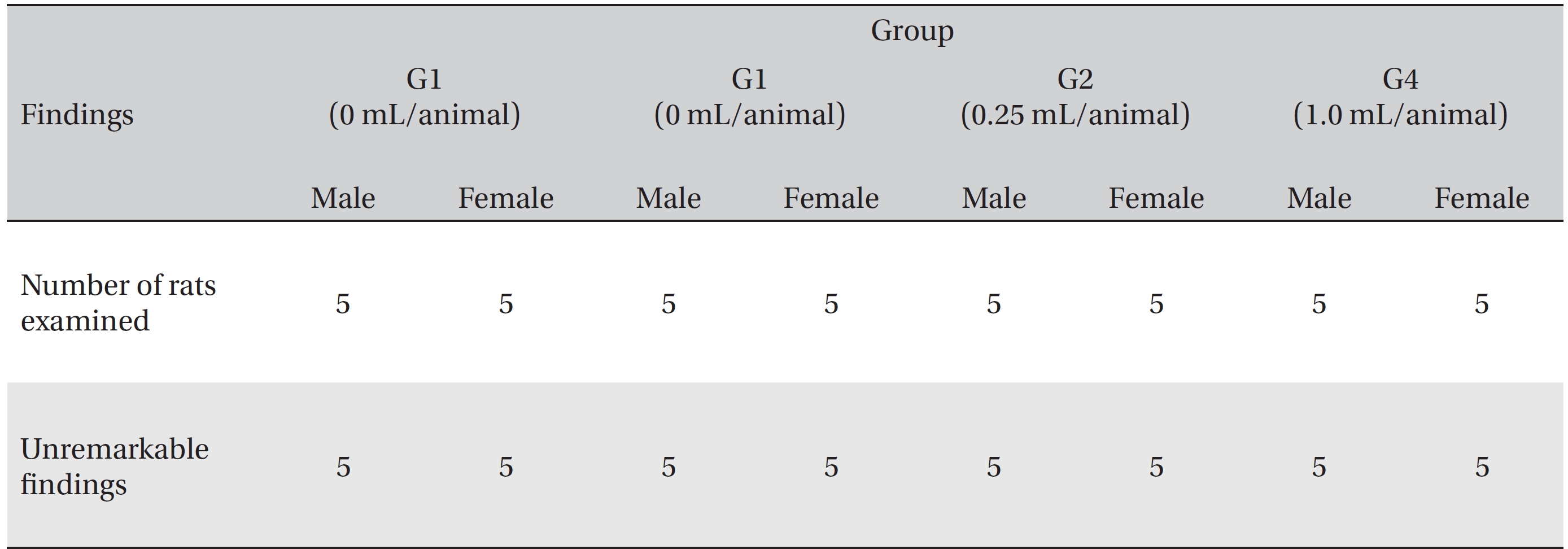
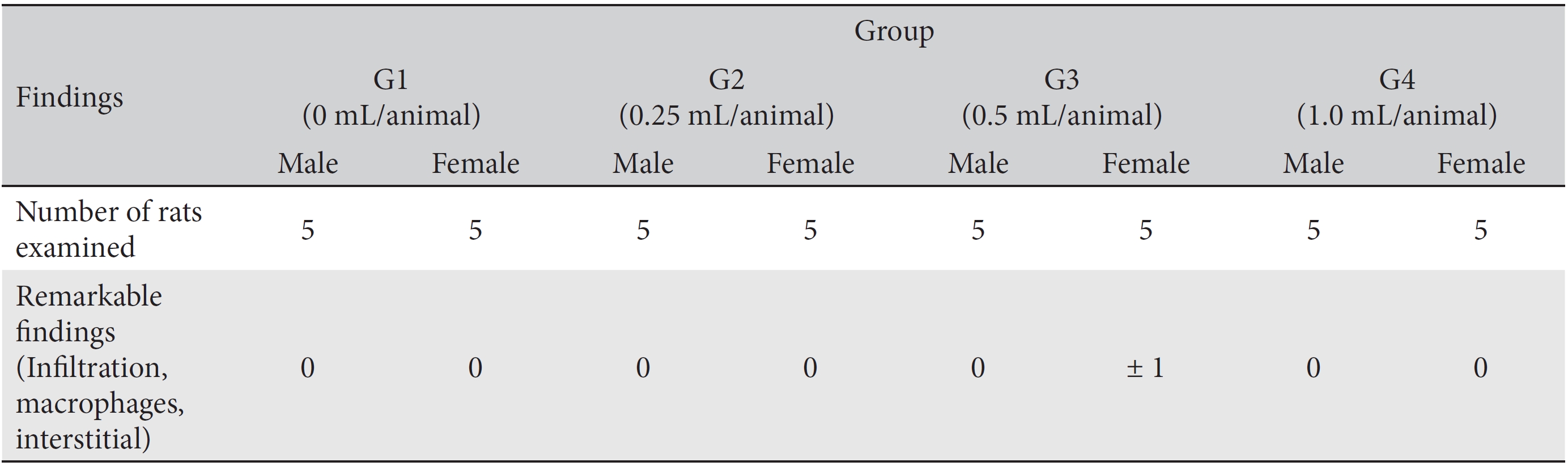
In this study, no deaths or abnormalities occurred in any of the groups (Tables 2,3). In addition, no changes in weight were observed in any of the groups (Table 4). Finally, no remarkable changes were observed on the hematological examination, the blood chemical test, the necropsy or the histopathological examination (Tables 5,6,7,8).
[Table. 2] Summary of mortalities
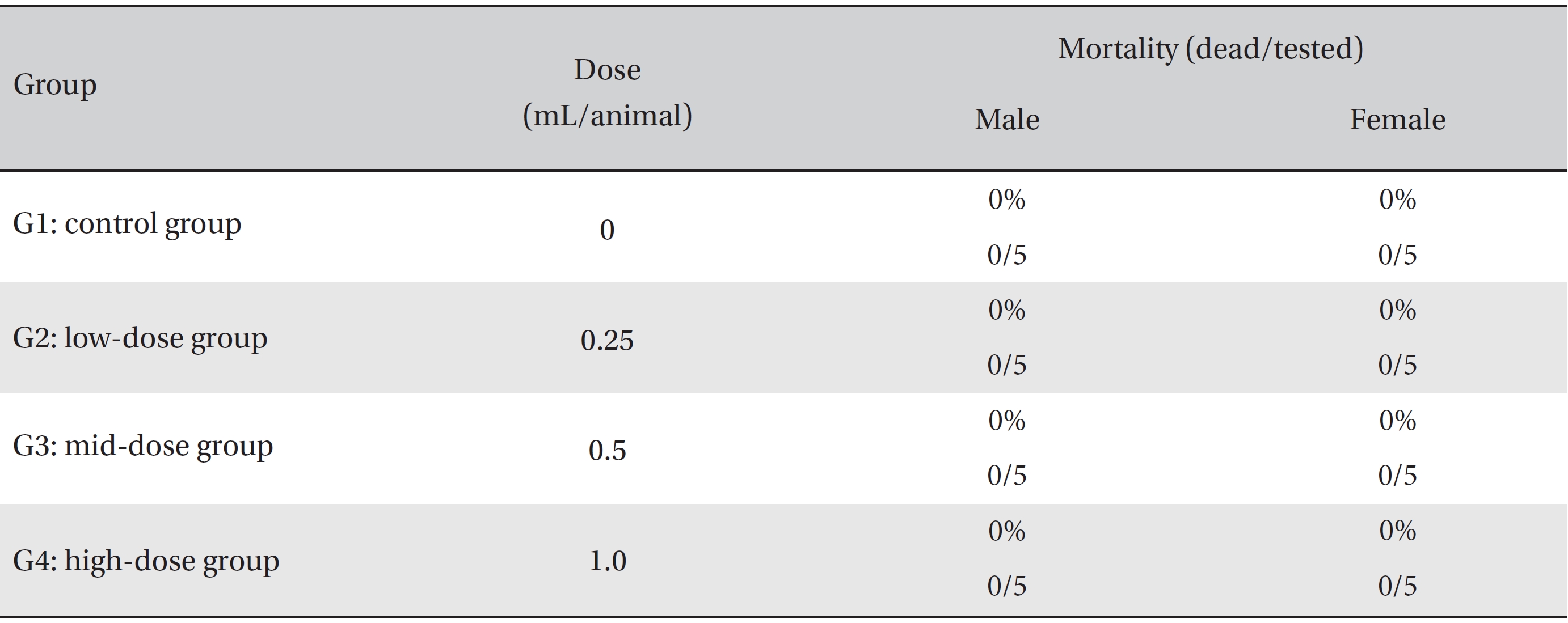
Summary of mortalities
[Table. 3] Summary of clinical signs
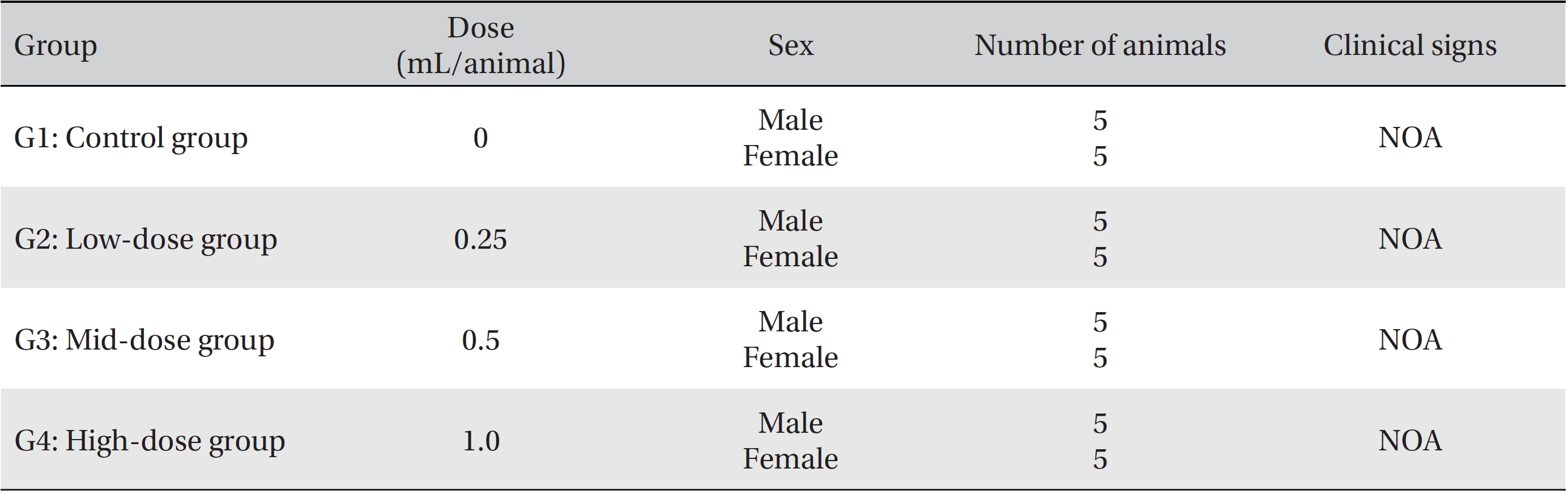
Summary of clinical signs
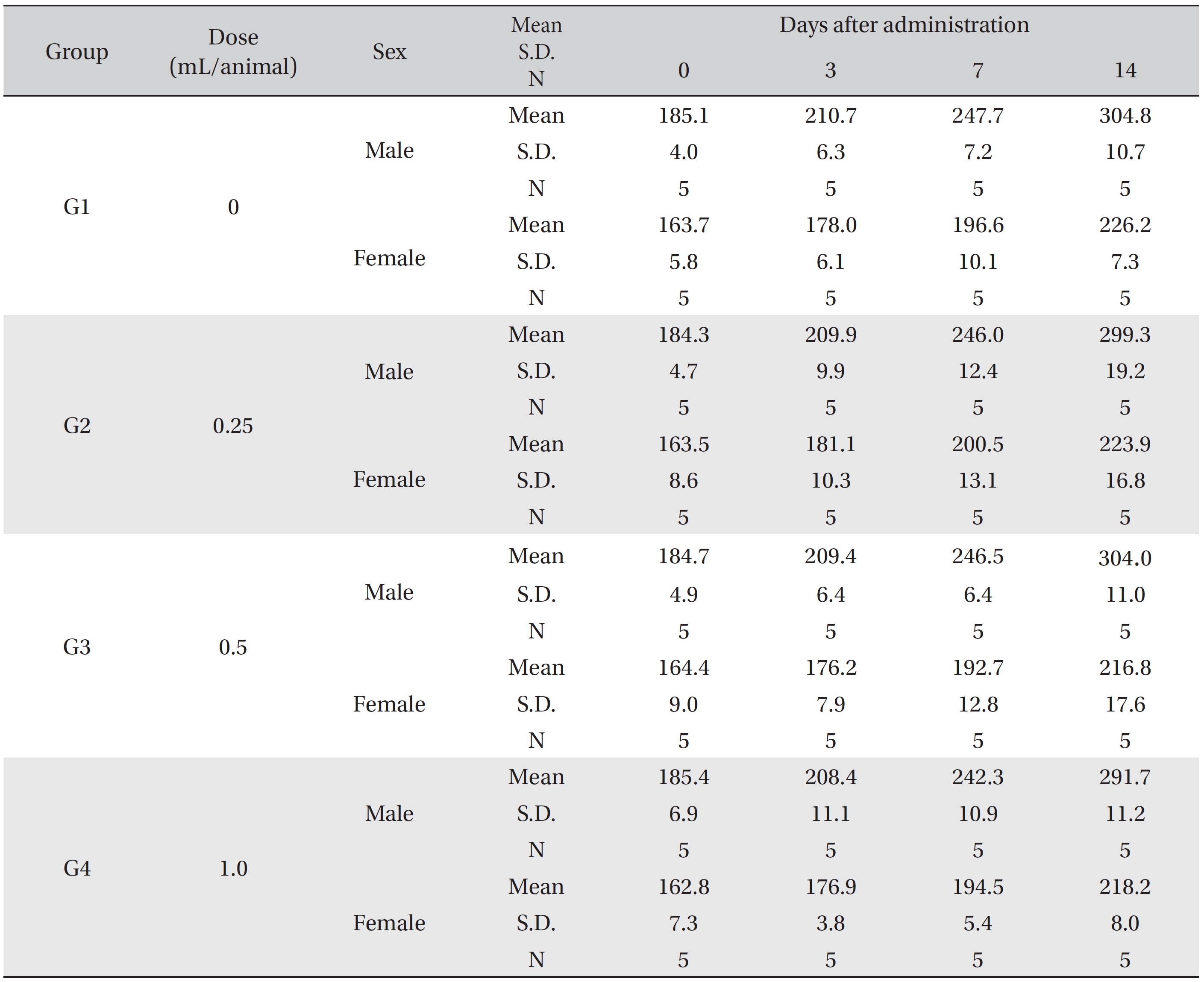
Mean body weights
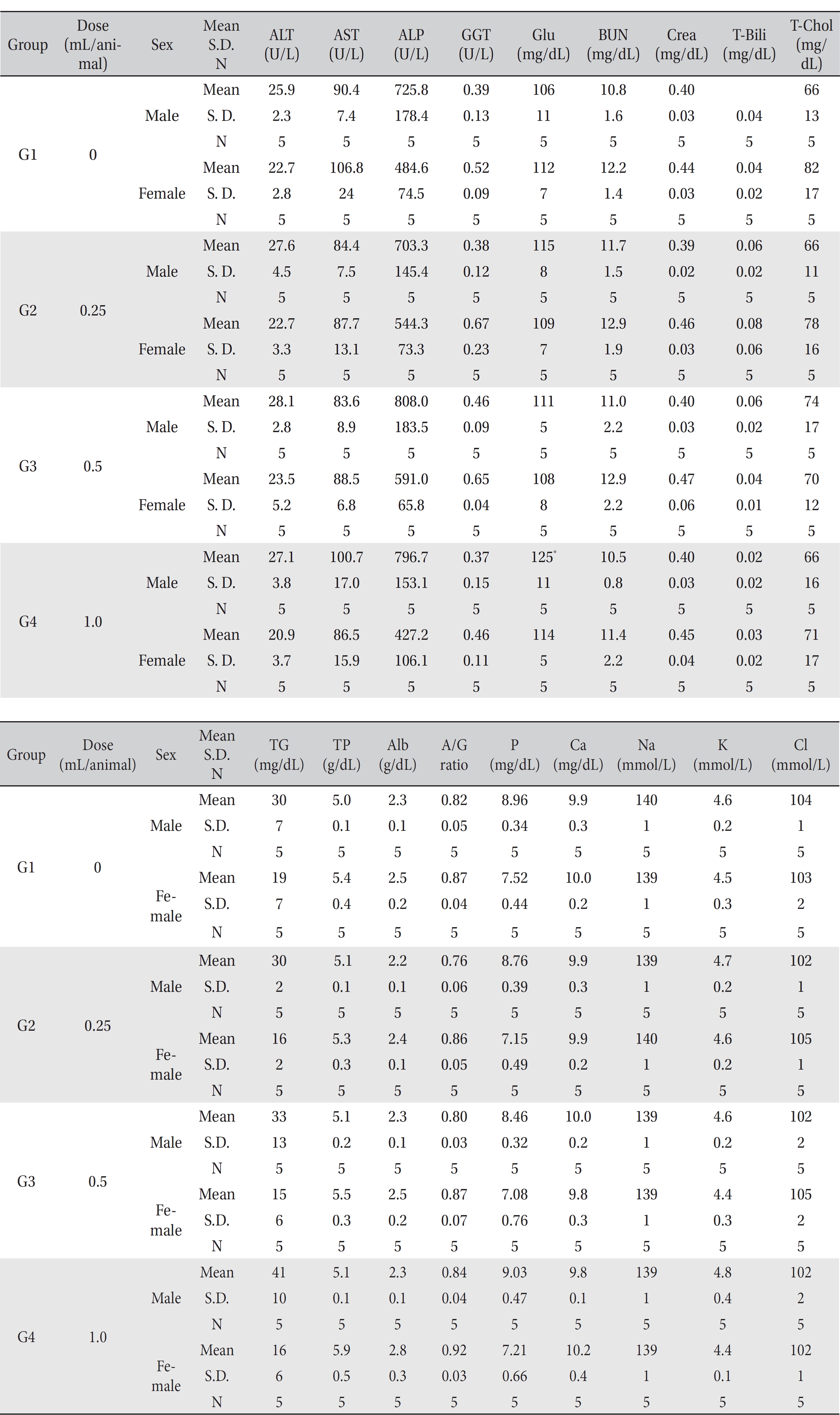
[Table. 5] Mean hematology parameters
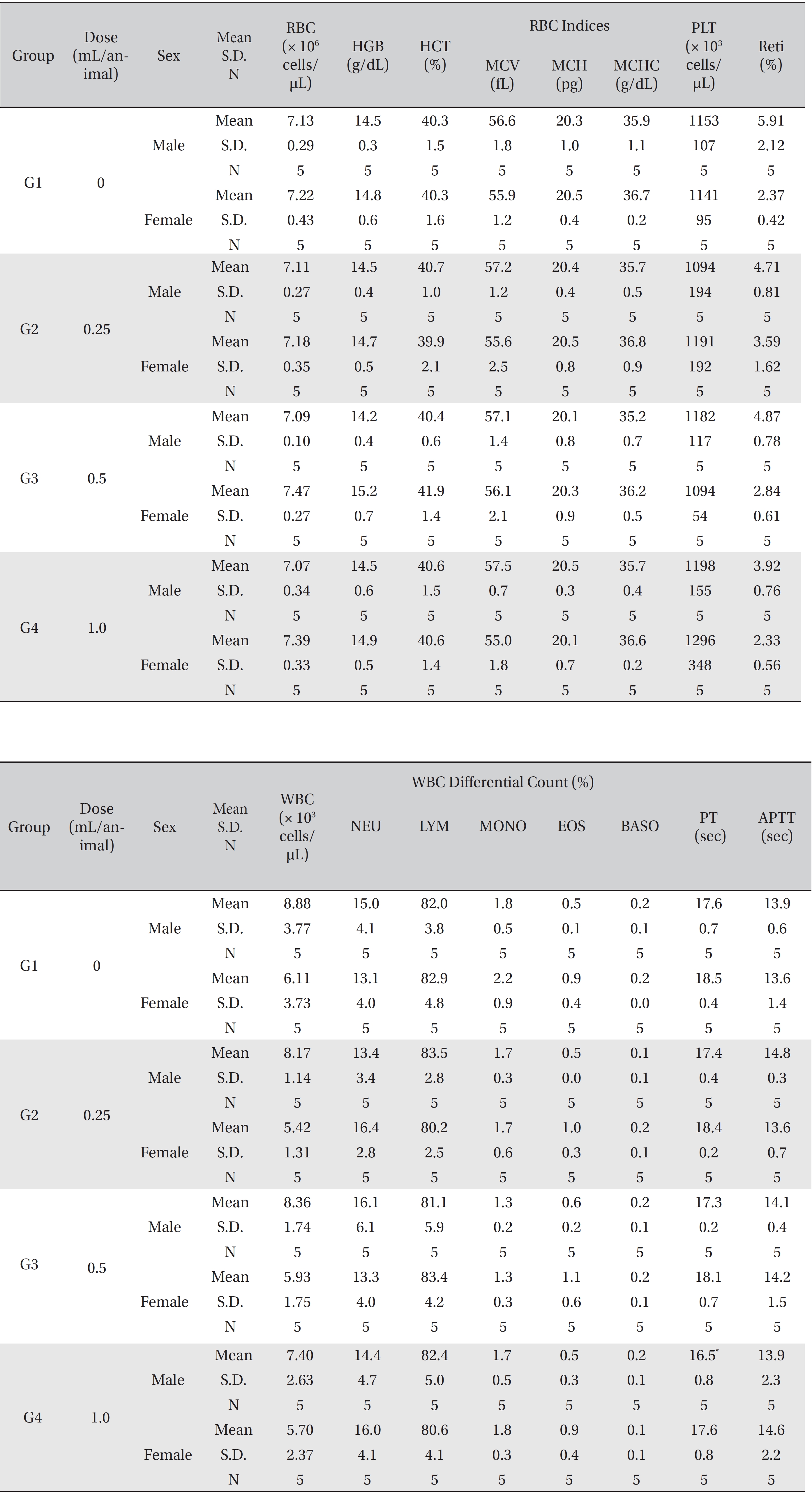
Mean hematology parameters
[Table. 6] Mean clinical chemistry
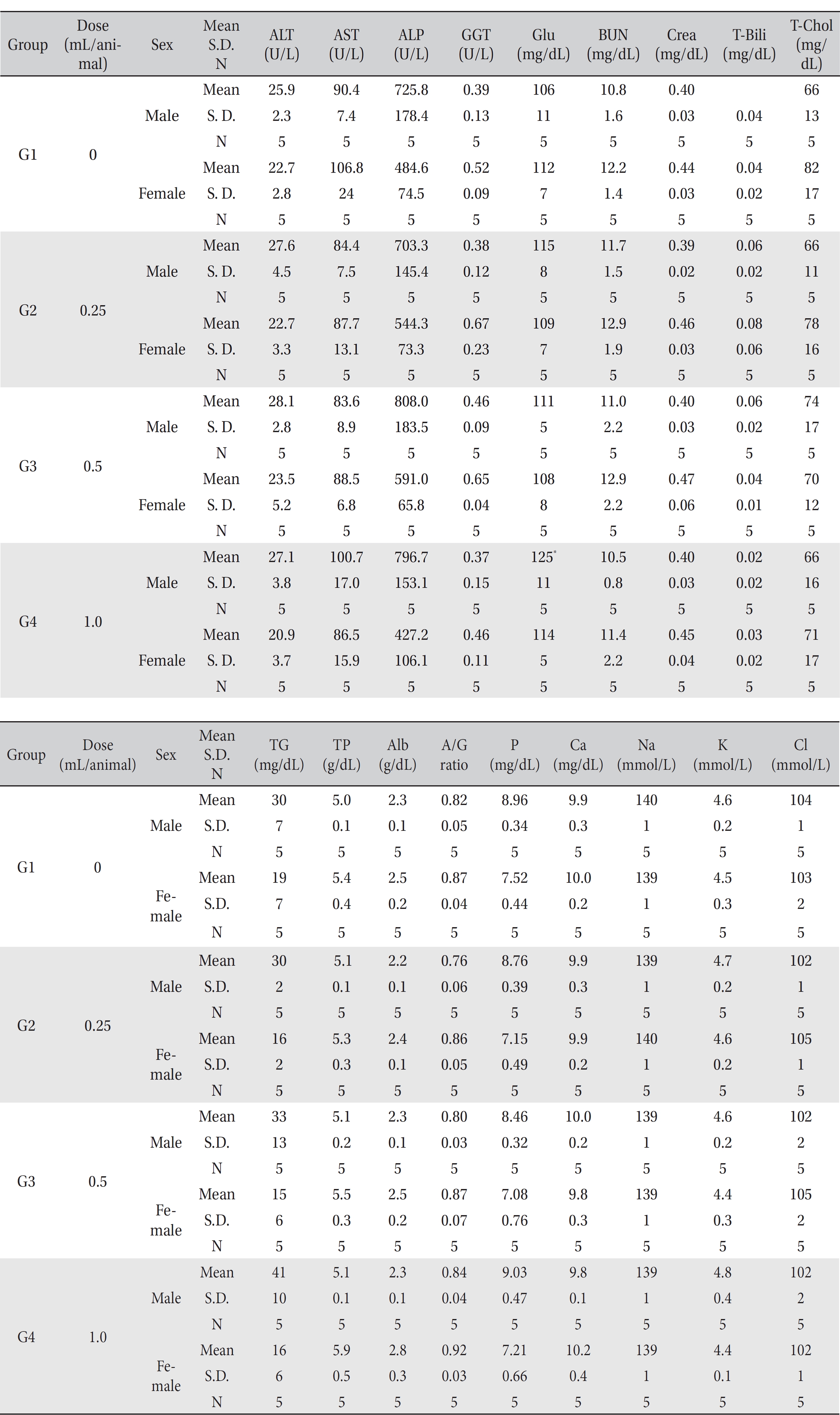
Mean clinical chemistry
[Table. 7] Summary of necropsy findings
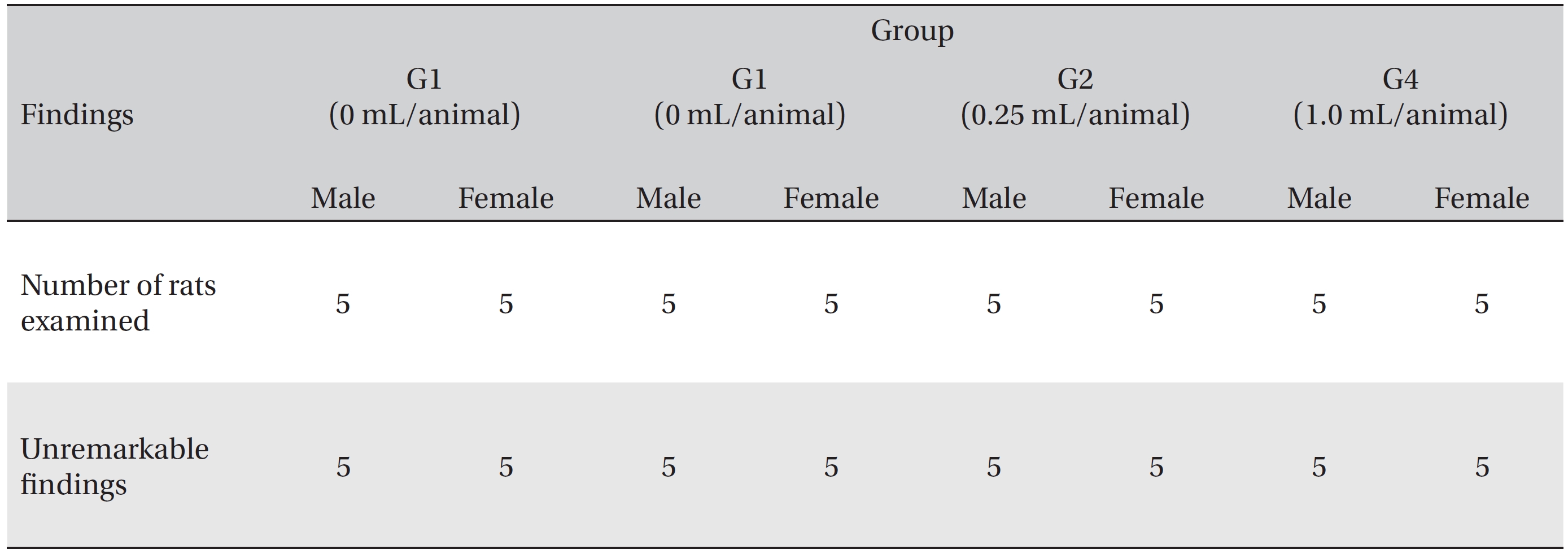
Summary of necropsy findings
[Table. 8] Histopathological findings
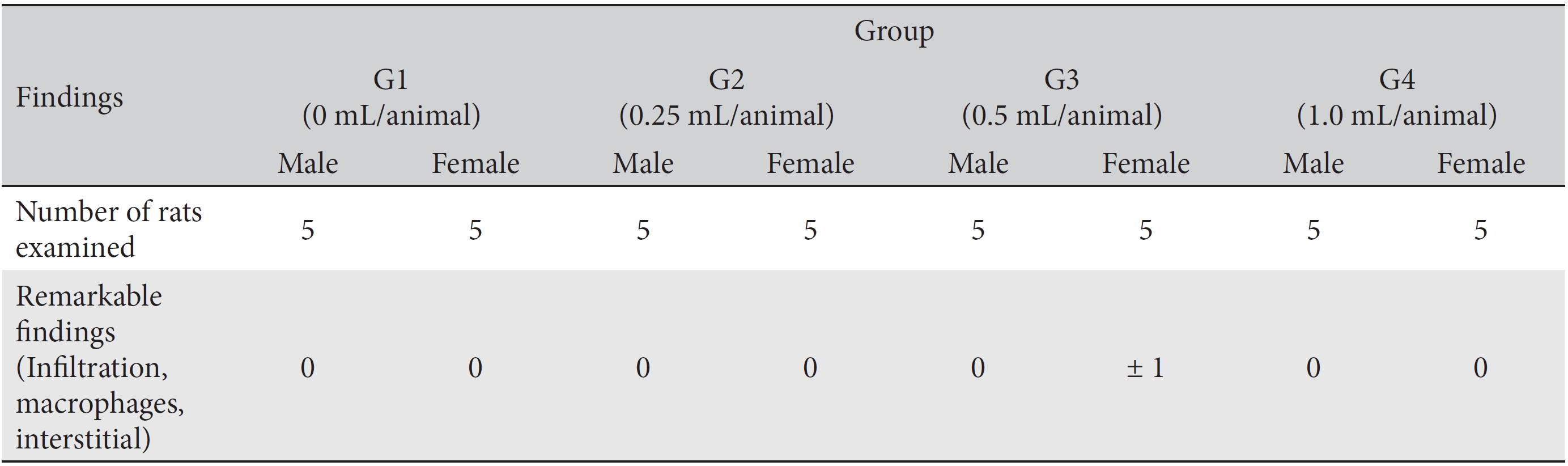
Histopathological findings
Eun-Bi San pharmacopuncture has been widely utilized in clinics. In spite of this, no clinical reviews on the effects of Eun-Bi San pharmacopuncture have been published, but there have been many studies on the component herbs of this pharmacopuncture have been published. Pharmacopuncture of
Thus, the component herbs of Eun-Bi San pharmacopuncture have been reported to have many effects on several disorders. Although it is used in clinics, safety studies on Eun-Bi San pharmacopuncture are insufficient, so more safety studies are needed. The toxicity study is an essential data base and is important for evaluating the safety of test substances in medications [11].
This study was carried out to provide objective safety data for Eun-Bi San pharmacopuncture. Doses of 0.25, 0.5 and 1.0 mL of Eun-Bi San pharmacopuncture were administered to the experimental groups, and a 1.0-mL dose of normal saline solution was administered to the control group. No deaths occurred, and no abnormalities were found in any of the four groups. No significant changes in the clinical signs, weights, hematologic examination results and blood chemical test results were observed; all results were within normal ranges. When the rats were dissected to check for abnormalities in organs and tissue, no significant histopathological findings were suggested.
To analyze the toxicity of Eun-Bi San pharmacopuncture, we need more study on its acute and chronic harmful effects and its relations with capacity reaction. Animal testing is the ultimate and basic way to conduct safety assessments [12]. The Korea Food & Drug Administration has testing protocol guidelines for the study of toxicity, and all experiments should be carried out following GLP regulations [13].
The results showed that treatment with 1.0-mL/animal of Eun-Bi San pharmacopuncture produced no changes in weight or in the results of hematological, blood chemical, and autopsy examinations. Because Eun-Bi San pharmacopuncture had no risks associated with its use in this study, Eun-Bi San pharmacopuncture administration can be considered to be a safe treatment; however, additional study is needed to confirm this result.
The results suggest that an intramuscular injection of 1.0-mL/animal of Eun-Bi San pharmacopuncture did not cause any changes in weight or in the results of hematological, blood chemical, and necropsy examinations. It also did not result in any mortalities. These results indicates that Eun-Bi San pharmacopuncture intramuscular injection can be used as a safe treatment.