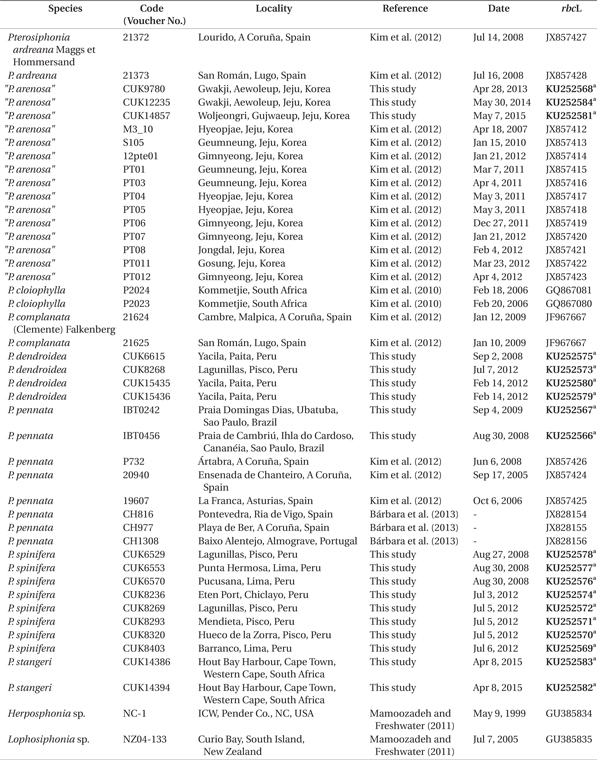
Pterosiphonia Falkenberg is the largest genus in the tribe Pterosiphonieae. On the basis of the type species, Pterosiphonia cloiophylla (C. Agardh) Falkenberg, Pterosiphonia is characterized by a bilaterally symmetrical thallus, prostrate and erect systems of indeterminate growth, erect axes having alternately distichous branches, a strongly compressed to flattened frond, and a congenital fusion of the proximal parts of the short laterals with the main axes (Maggs and Hommersand 1993, Kim et al. 2012). Twenty-one species have been described in the genus, and they occur in temperate to tropical regions of the Atlantic and Pacific Oceans (Kim et al. 2012, Guiry and Guiry 2014).
Twelve Pterosiphonia species have been reported from the Pacific Ocean. Of them, Pterosiphonia pennata (C. Agardh) Sauvageau was originally described from the Mediterranean Sea and later reported worldwide, including in Korea and Peru (Dawson et al. 1964, Norris and Aken 1985, Lee et al. 1992, Maggs and Hommersand 1993, Hoffmann and Santelices 1997, Abbott 1999, Womersley 2003). Maggs and Hommersand (1993) pointed out, however, that the distribution of P. pennata is difficult to assess at present because of confusion among groups of morphologically similar species. Pterosiphonia spinifera (Kützing) Ardré was originally described as Polysiphonia spinifera from the Peruvian Coast (Kützing 1843). Ardré (1967) transferred Polysiphonia spinifera Kützing to Pterosiphonia after examination of the type material and showed that the features of Pterosiphonia pennata in Peru corresponded to those of Pterosiphonia spinifera. Pterosiphonia spinifera is currently accepted as a species within Pterosiphonia (Silva et al. 1996).
Recently, Kim et al. (2012) described Pterosiphonia arenosa M. S. Kim et B. Kim as a new species based on material previously called P. pennata from Jeju Island, Korea, after investigating specimens identified as P. pennata from Korea and from Spain near the type locality. Kim et al. (2012) distinguished P. arenosa from P. pennata by the absence of vegetative trichoblasts and the straight arrangement of the tetrasporangia, and recognized P. arenosa as endemic to the northwest Pacific region.
We collected samples that fit the descriptions of Pterosiphonia spinifera and P. arenosa from their type localities in Peru and Korea. We also compared these samples with the isotype specimens of P. arenosa based on their morphology and on rbcL molecular data. In this study, we conclude that P. spinifera and P. arenosa are conspecific and P. arenosa is a later synonym of P. spinifera.
Samples were collected along the coasts of Korea and Peru from 2008 to 2015. The samples were preserved in 4-5% formalin / seawater for morphological examination, and in silica gel for molecular analysis. Material was stained with 1% aqueous aniline blue acidified with 0.1% diluted HCl for microscopic observations. Photomicrographs were taken using an Olympus microscope (BX51TRF; Olympus, Tokyo, Japan) equipped with an Olympus DP71 camera. We selected 25 plants from five tufts for the determination of quantitative characters, and we calculated the means and standard deviations of these characters. Voucher specimens were deposited in the herbarium of Chosun University (CUK), Korea and in the National Institute of Biological Resources (NIBR), Incheon, Korea.
Genomic DNA was extracted from silica-gel-dried samples using the NucleoSpin Plant II kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed in a final volume 20 μL using 2.5 μL of genomic DNA, 2 mM of dNTP mix, 1× reaction buffer, 0.25-0.5 pmol of forward and reverse primers, and 0.01 U of TOP DNA polymerase (Bioneer, Daejon, Korea). The rbcL gene was amplified using the primer combination F7-R753 and F645-Rrbcst (Freshwater and Rueness 1994, Bustamante et al. 2012, 2013) and purified with the PCRquick-spin PCR product purification kit (iNtRON Biotechnology, Inc., Seongnam, Korea). Cycle sequencing was performed with the amplification primers. Sequencing was performed using the ABI Prism 3100 Genetic Analyzer (PE Applied Biosystems, Foster City, CA, USA). Nineteen new rbcL sequences were obtained from Pterosiphonia arenosa, P. dendroidea (Montagne) Falkenberg, P. pennata, P. spinifera, and P. stangeri (J. Agardh) Falkenberg (deposited in EMBL / GenBank) (Table 1). The rbcL sequence obtained in the present study and others obtained from GenBank were aligned using ClustalW (Thompson et al. 1994). Lophosiphonia sp. (GU385835) and Herposiphonia sp. (GU385834) were selected as the outgroups. Phylogenetic and molecular evolutionary analyses using the maximum likelihood analysis were conducted with 1,000 bootstrap replications in MEGA6 under the GTR + Γ + I model (Tamura et al. 2013). A bayesian inference was performed using MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001, Ronquist and Huelsenbeck 2003). The Markov chain Monte Carlo (MCMC) runs were performed for four million generations, each with one cold chain and three heated chains, using the GTR + Γ + I evolutionary model (sampling and printing every 1,000 generations). Summary trees were generated using a burn-in of 25%.
Our morphological and genetic results indicate that Pterosiphonia spinifera from Peru and P. arenosa from Korea are identical, and they differ from P. pennata specimens collected near the type locality in Europe. We present below a detailed description of P. spinifera along with recommended nomenclatural changes.
Basionym. Polysiphonia spinifera Kützing 1843.
Heterotypic synonym. Pterosiphonia arenosa M. S. Kim & B. Kim.
Type locality. Peru.
Specimens examined. CUK6529 (Aug 27, 2008, Lagunillas, Pisco, Peru); CUK6553 (Aug 30, 2008, Punta Hermosa, Lima, Peru); CUK6570 (Aug 30, 2008, Pucusana, Lima, Peru); CUK8236 (Jul 3, 2012, Eten Port, Chiclayo, Peru); CUK8269 (Jul 5, 2012, Lagunillas, Pisco, Peru); CUK8293 (Jul 5, 2012, Mendieta, Pisco, Peru); CUK8320 (Jul 5, 2012, Hueco de la Zorra, Pisco, Peru); CUK8403 (Jul 6, 2012, Barranco, Lima, Peru); CUK9780 (Apr 28, 2013, Gwakji, Aewoleup, Jeju, Korea); CUK12234-CUK12235 (May 30, 2013, Gwakji, Aewoleup, Jeju, Korea); CUK14857 (May 7, 2015, Woljeongri, Gujwaeup, Jeju, Korea); and NIBRAL0000133084-NIBRAL0000133086 (Dec 27, 2011, Gimnyeong, Jeju Island, Korea, the same specimens previously reported as isotypes of P. arenosa by Kim et al. 2012).
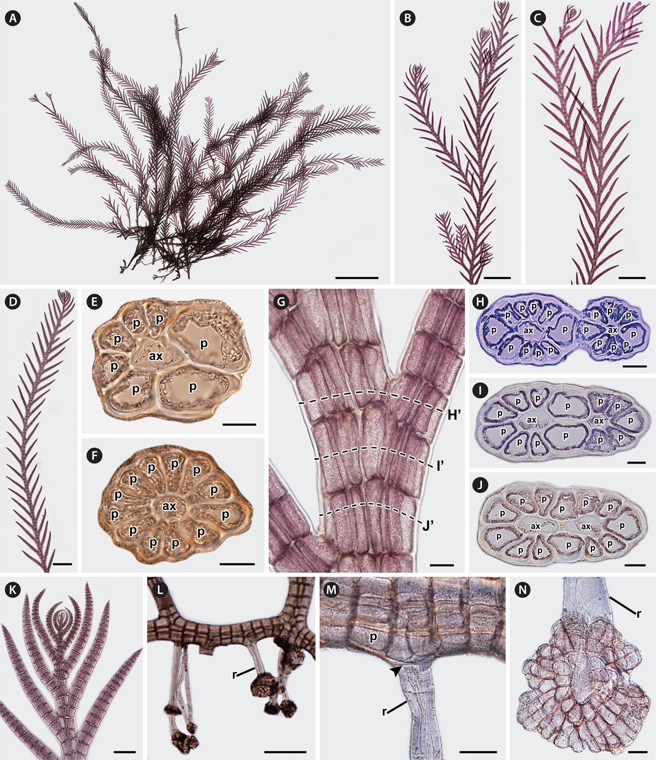
Description. Thalli of P. spinifera are large, 1.9-5.9 cm in height (Fig. 1A), purplish red in color, and associated with other filamentous species. Thalli form large tufts, usually attached to rock surfaces or growing as epiphytes on the genicule of Corallina. Dorsiventral thalli are composed of prostrate and erect systems (Fig. 1A). Prostrate systems are cylindrical and composed of extensive and entangled exogenous indeterminate axes, laterally or dorsally branched at irregular intervals. Segments of prostrate axes are 68.85 ± 12.02 μm in length and 94.91 ± 10.01 μm in diameter, being 1.37 times broader than long (0.72 ± 0.18 in L / D). Erect systems with indeterminate growth (Fig. 1B & C) are cylindrical at the base becoming compressed near the branches. The main erect axes are robust and produce exogenously alternate-distichous branches of determinate growth of first order (Fig. 1D) every two segments, and rarely of second order (Fig. 1B & C). They are composed of 7-12 pericentral cells, ecorticated throughout (Fig. 1E-G), and sparsely branched at the base to abundantly branched above (Fig. 1B & L). Branches of erect axes have congenital fusions of the proximal parts of 1-1.5 segments with bearing axes of first order and second order (Fig. 1G-J). In young erect axes (Fig. 1K), laterals are formed by short segments and slightly curved in the direction of the main axes, and their apical cells are completely separated from the parental axis and not in congenital fusion. In older erect axes (Fig. 1G), segments are 101.39 ± 7.75 μm in length and 141.22 ± 8.05 μm in diameter, being 1.40 times broader than long (0.71 ± 0.06 in L / D) and in congenital fusion with laterals. Apical cells are prominent, (8.85 ± 1.51 μm) × (5.42 ± 0.72 μm) in size, transversely or oblique divided, and dome shaped. Vegetative trichoblasts and scar cells are absent. Rhizoids are scattered and ventrally produced, one to four per segment, from the distal end of pericentral cells of prostrate axes (Fig. 1L). They are cut off from the pericentral cells (Fig. 1M). Rhizoids are unicellular in younger stages but when mature they have multicellular terminations (Fig. 1N) and are 30.39 ± 7.18 μm in diameter and 328.70 ± 135.80 μm in length.
In female gametophytes (Fig. 2A & B), erect axes are densely branched in their upper parts. Procarps are formed laterally and subapically to the first order of determinate branches on the erect axes (Fig. 2C) and composed of a supporting cell bearing a 4-celled carpogonial branch and a basal sterile cell (Fig. 2D). Cystocarps are ovoid when mature (Fig. 2E), 282.01 ± 71.62 μm in height and 236.75 ± 51.21 μm in diameter. In tetrasporangial plants (Fig. 2F), tetrasporangia are tetrahedrally divided and (27.47 ± 8.85 μm) × (25.57 ± 3.52 μm) in size and formed in the determinate branches of second order erect axes (Fig. 2G). Tetrasporangial branches are swollen and linear (Fig. 2G). Development of tetrasporangia follows a straight arrangement (Fig. 2G). The fertile segments have 8-10 pericentral cells. The fertile pericentral cell is produced into a stalk cell, which develops into the tetrasporangium and two presporangial cover cells. One tetrasporangium is produced from a single segment and surrounded by one or two extra postsporangial cover cells (Fig. 2H). Male gametophytes were not found.
[Fig. 2.] Reproductive structures of Pterosiphonia spinifera from Peru. (A & B) Female gametophyte bearing cystocarps. (C) Apex showing cystocarp (arrowhead) formed on adaxial side of alternate-distichous branches of the second order. tb, trichoblast. (D) Procarp with a 4-celled carpogonial branch (1-4, sequence cells of carpogonial branch) on supporting cell (su). st, sterile cell; tb, trichoblast. (E) Mature cystocarp showing ovoid shape. (F) Tetrasporangial plant. (G) Apex showing the arrangement of tetrasporangia (t) in a straight series on alternate-distichous branches of the second order. (H) Determinate branches showing tetrasporangia (t) surrounded by two presporangial cover cells (arrowheads) and one or two postsporangial cover cells (arrows). Scale bars represent: A, 1 mm; B, 500 μm; C & H, 20 μm; D, 10 μm; E, 50 μm; F, 2 mm; G, 100 μm.
Habitat. Plants grow in the intertidal zone, forming large and very robust tufts of 10 cm2. They are found from the northern to the southern coast of Peru and around Jeju Island in Korea in sheltered to wave-exposed areas, and attached to sand-covered rocks or as epiphytes on Corallina. Sometimes P. spinifera was found in association with P. dendroidea and Streblocladia camptoclada (Montagne) Falkenberg.
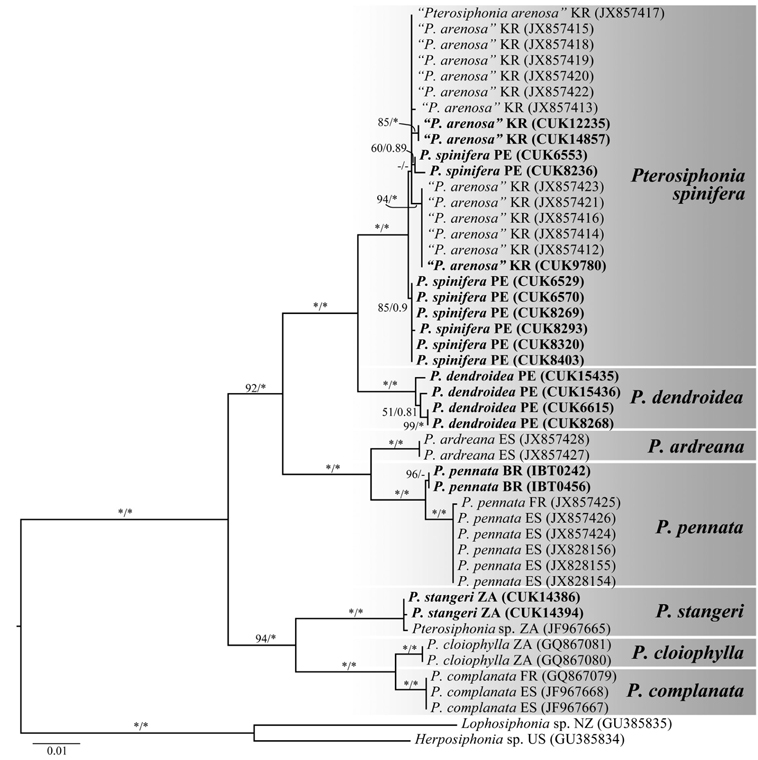
Molecular analysis. A 1,394-bp portion of the rbcL gene sequenced from seven species of Pterosiphonia was used in the analysis. Phylogenetic analyses revealed that Pterosiphonia spinifera was placed in the same clade with samples of P. arenosa from Korea and was clearly separated from P. pennata collected from Spain, France, and Brazil (Fig. 3). The sequence divergences between P. spinifera and P. arenosa and between P. pennata from Brazil and P. pennata from Europe were 0.2-0.4% and 0.5%, respectively.
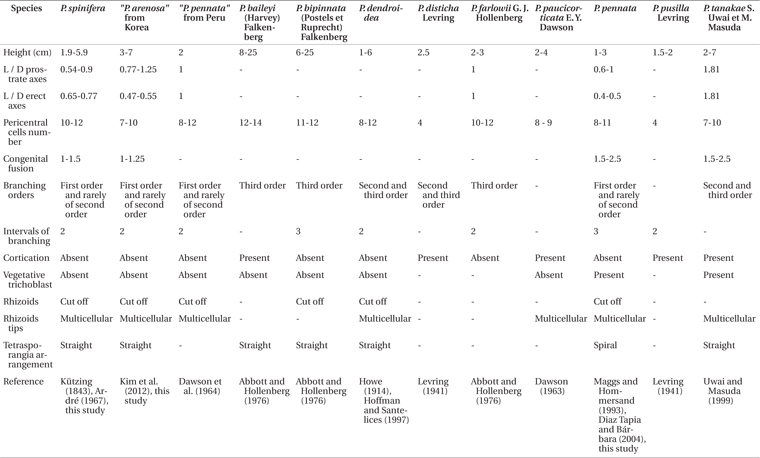
Our specimens from Peru and Korea correspond to the Pterosiphonia spinifera previously reported by Kützing (1843) and Ardré (1967). Kützing (1843) originally described Polysiphonia spinifera with the following features from Peru: pinnate branches, spine-like lateral branches, and 10 pericentral cells. Ardré (1967) added other detailed characters after observation of the type specimens: simple branches with some branches of second order, coalescence of almost two segments, and 10-12 pericentral cells. Although Kützing (1843) did not mention a specific area in Peru for the type locality, all our materials collected along the coastline of Peru appear identical to P. spinifera in Kützing’s original description and Ardré’s detailed morphology (Table 2).
In the South American Pacific, Pterosiphonia pennata was reported from San Lorenzo Island and La Libertad, Peru, by Taylor (1947). After this record, P. pennata has been reported as a common species in South America including in Peru (e.g., Dawson et al. 1964, Ramírez and Santelices 1991, Hoffmann and Santelices 1997), because P. pennata has been recognized on the basis of general characters such as having an ecorticate thallus, erect axes with alternate-distichous branches, and 9-11 periaxial cells (Maggs and Hommersand 1993). Although P. pennata is similar to P. spinifera in these characters, P. pennata can be distinguished from P. spinifera by a spiral arrangement of tetrasporangia and the presence of trichoblasts (Maggs and Hommersand 1993, Díaz Tapia and Bárbara 2004, Kim et al. 2012). We did not collect any samples matching P. pennata along the coast of Peru. The reports of P. pennata from Peru seem to be misidentifications based on Pterosiphonia spinifera from the Peruvian coast (Table 2).
Pterosiphonia arenosa was characterized by the following features: 1) thalli composed of erect and prostrate axes of indeterminate growth, 2) ecorticate thalli, 3) 7-10 pericentral cells, 4) branches formed every two segments, 5) determinate branches borne in an alternate-distichous pattern, 6) 1-1.25 segments coalesced with main axis, 7) absence of trichoblasts, and 8) straight arrangement of tetrasporangia (Kim et al. 2012). P. arenosa was described based on materials known as P. pennata from Korea after comparison of P. arenosa with P. pennata samples from near the type locality (Kim et al. 2012). However, the characters of P. arenosa (Fig. 4A-Q) appear to be identical to those of P. spinifera (Table 2). In addition, our molecular phylogenetic analyses using rbcL gene sequences show that P. arenosa and P. spinifera are the same species with low sequence divergence (below 0.4%).
[Fig. 4.] Vegetative and reproductive structures of Pterosiphonia arenosa from Korea. (A) Habit of the vegetative plant showing entangled prostrate axes and erect axes. (B & C) Erect axes showing simple laterals and laterals of the first order. (D & E) Cross-section views of apex (D) and erect axes (E) showing 7-9 pericentral cells. ax, axial cell; p, pericentral cell. (F) Erect axes showing congenital fusions of proximal parts with bearing axes in the branches. (G-I) Cross-section views of the erect axes labeled in (F) showing congenital fusions. (J) Apex showing laterals slightly curved in the direction of the main axes. (K) Rhizoid (r) scattered and produced from the distal end of pericentral cells. (L) Rhizoid (r) cutting off (arrowhead) from pericentral cells. (M) Cross-section view showing rhizoids (r) cutting off (arrowhead) from pericentral cells. (N) Tetrasporangial plant. (O) Apex showing the arrangement of tetrasporangia (t) in a straight series in alternate-distichous branches of the second order and determinate growth. (P) Determinate branches showing tetrasporangia (t) surrounded by two presporangial cover cells (arrowheads) and one postsporangial cover cell (arrow). (Q) Determinate branches showing tetrasporangia (t) surrounded by two presporangial cover cells (arrowhead) and two postsporangial cover cells (arrows). Scale bars represent: A & N, 5 mm; B, 1 mm; C, 500 μm; D & E, 10 μm; F & O, 50 μm; G- I, L, M, P & Q, 20 μm; J, 100 μm; K, 200 μm.
The absence of trichoblasts, the simple axes with first order branches, and the straight arrangement of the tetrasporangia may be used as important characters for identification of Pterosiphonia spinifera. Although trichoblasts have been shown to occur rarely, seasonally, or only in connection with sexual reproductive structures in some polysiphonous genera (Hollenberg 1942), the occurrence of trichoblasts in the vegetative structures of Pterosiphonia has been shown to have taxonomic value in delimiting species (Maggs and Hommersand 1993, Uwai and Masuda 1999). The lack of vegetative trichoblasts distinguishes P. spinifera from P. pennata. The alternate-distichous branching pattern is common in all species of Pterosiphonia. This branching pattern has branches of first to third orders and it has shown consistency (Ardré 1967). The simple axes with first order branches in P. spinifera distinguish it from other species with second or third order branches (e.g., P. dendroidea) (Dawson et al. 1964). The arrangement of the tetrasporangia has been considered a consistent character for recognizing species in Pterosiphonia (Maggs and Hommersand 1993, Uwai and Masuda 1999). Most Pterosiphonia species, including P. spinifera, have a straight arrangement of tetrasporangia, except P. pennata, which has a spiral arrangement of tetrasporangia (Díaz Tapia and Bárbara 2004).
In conclusion, Pterosiphonia spinifera from Peru was identical to P. arenosa from Korea in morphology, while it was clearly distinguished from P. pennata in Europe (Table 2). There was no evidence to consider P. arenosa and P. spinifera as different entities after our morphological comparisons. In addition, our phylogenetic analysis of rbcL confirms the similarities between P. spinifera and P. arenosa. Thus, P. arenosa should be synonymized with P. spinifera on the basis of the principle of priority (Article 11, International Code of Botanical Nomenclature). Our study confirms the wide occurrence of P. spinifera in the western and eastern Pacific Ocean. In addition, although Pterosiphonia spinifera has been reported in Brazil by Yoneshigue and Villaça (1986), our phylogenetic analysis reveals that P. spinifera reported in Brazil should be referred to P. pennata.