The family Tachidiidae Boeck, 1896 is a small group comprising 16 species of six genera (Tran and Chang, 2012). They are mainly characterized by nuchal organs, reduced setation of the maxilliped, and a single plate-like P5 in both sexes (Seifried, 2003). They inhabit mainly benthic from euryhaline environment (Chang, 2008). From Korean brackish waters, the following five species belonging to this family have been described:
Within the family Tachidiidae, the genus
During a study on harpacticoid fauna of Korea, the authors encountered specimens of
Samples were collected from mudflat of intertidal zone by using a 63 μm mesh size sieve. Specimens were fixed initially with 99.9% ethyl alcohol. Before dissection, habitus was drawn and observed from whole specimens mounted in lactophenol. Their appendages were dissected using tungsten needles under a stereomicroscope (Discovery. V8; Carl Zeiss, Göttingen, Germany). Each appendage and urosome were mounted in lactophenol on slides and sealed with Canada balsam. All line drawings were made using a drawing tube attached to a light microscope (Olympus BX53; Olympus, Tokyo, Japan) equipped with differential interference contrast. Materials examined in this study were deposited at the National Institute of Biological Resources (NIBR), Incheon, Republic of Korea.
The terminology for description follows that of Huys and Boxshall (1991). Abbreviations used in the text are as follows: P1-P6, first to sixth thoracopods; exp(enp)-1 (-2,-3), the proximal (middle, distal) segment of ramus.
>
?
Material examined. South Korea: 4♀♀, 2♂♂, Jeollanam-do, Jindo-gun, Uisin-myeon, Chosa-ri (34°24′55.55″N, 126°19′32.59″E) on 3 May 2015; 1♀ (NIBRIV0000470365) dissected on 14 slides; 1♂ (NIBRIV0000470366) dissected on eight slides; 1♀ (NIBRIV0000470367) dissected on seven slides; 1♂ (NIBRIV0000470368) dissected on eight slides; 1♀ (NIBRIV0000470369) dissected on nine slides; 1♀ (NIBRIV0000470370) dissected on 13 slides.
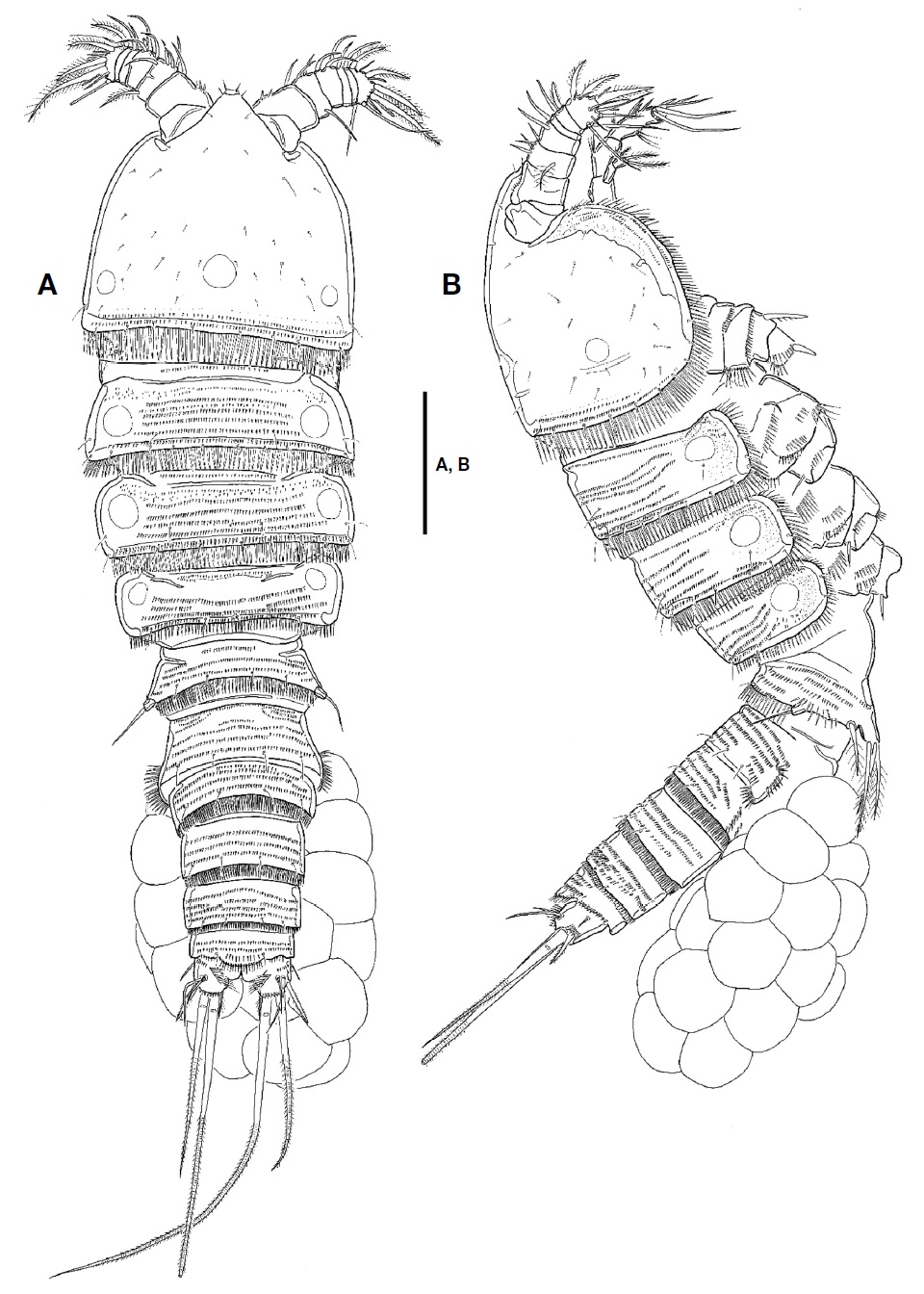
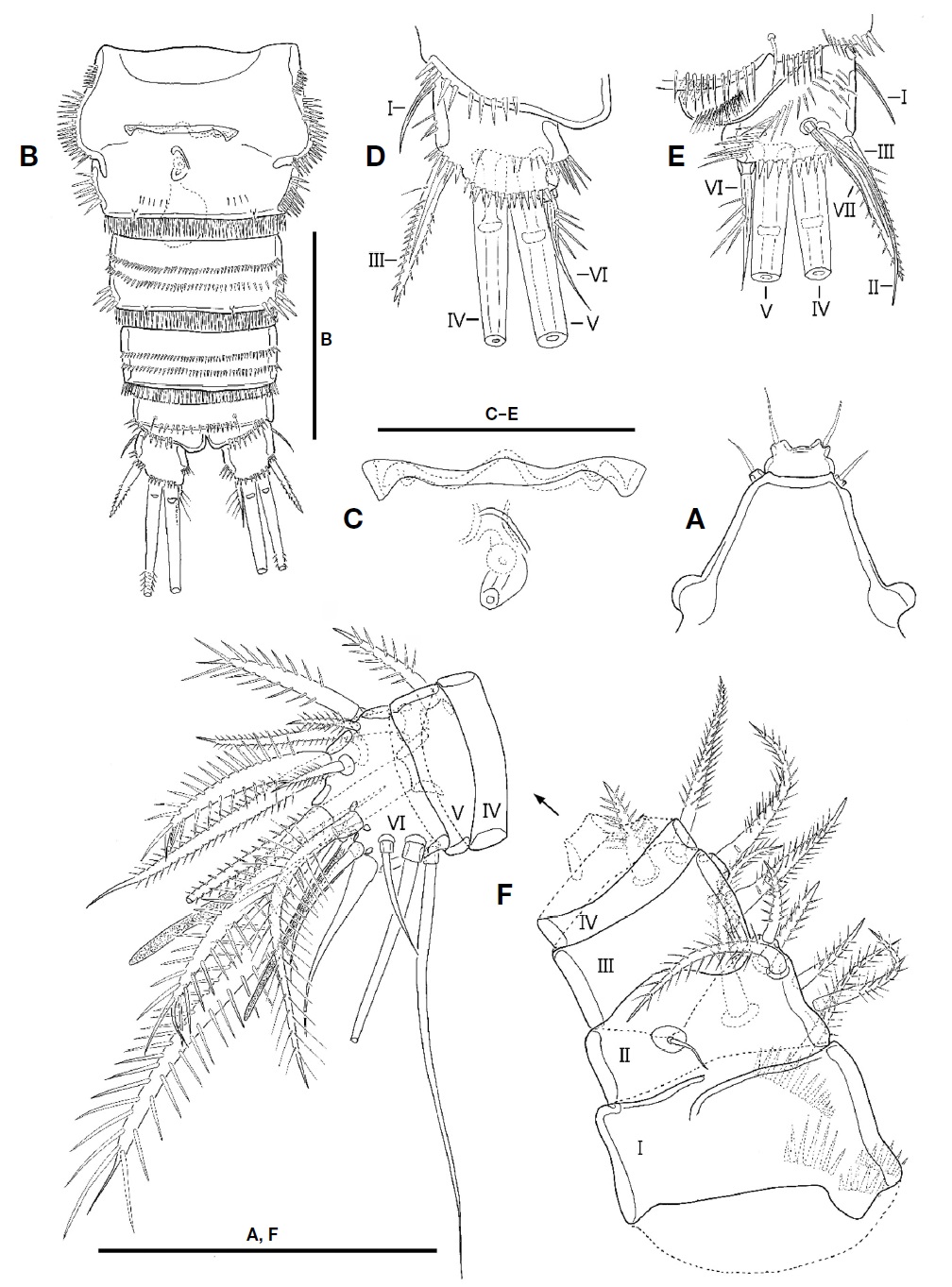
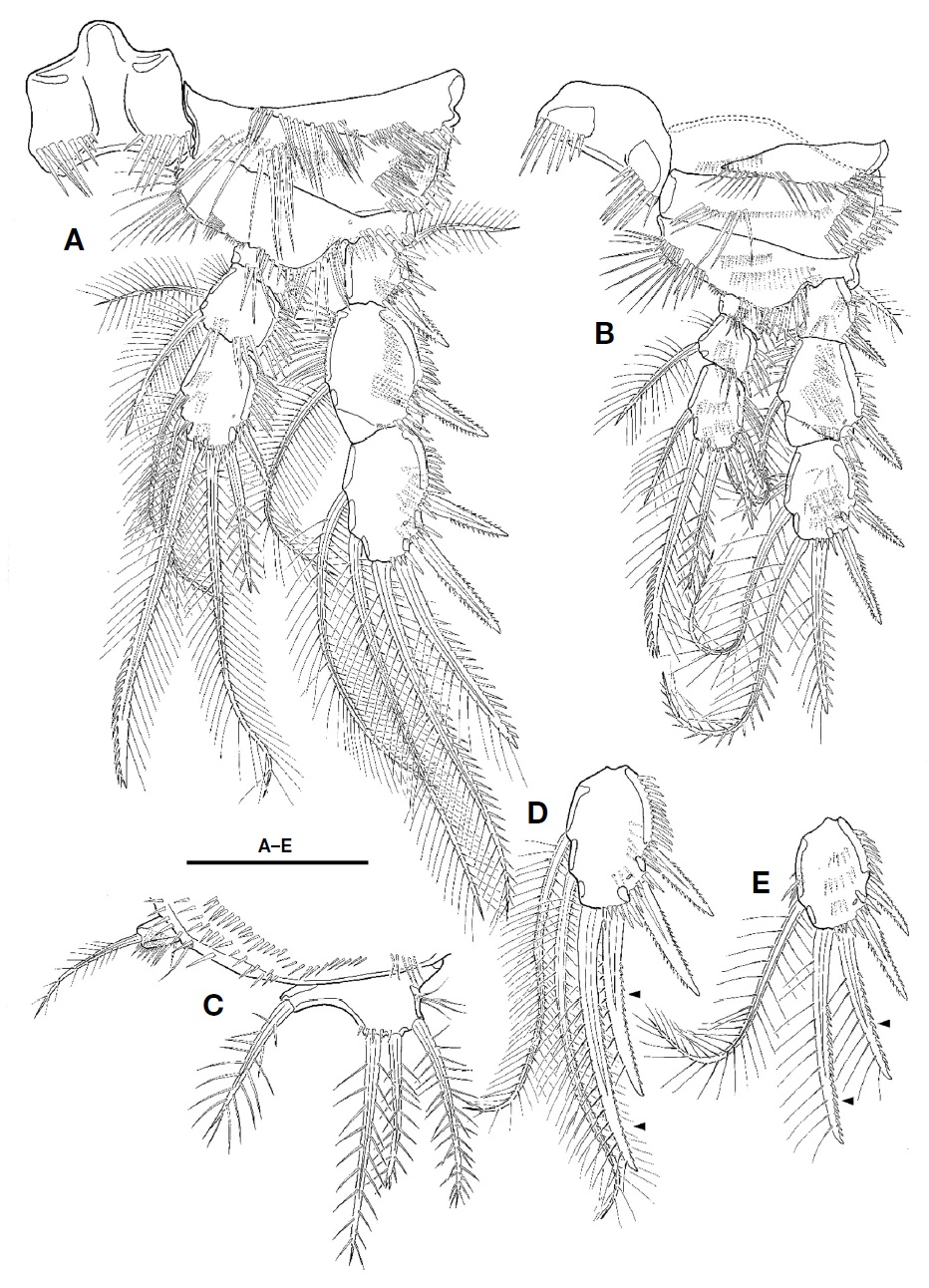
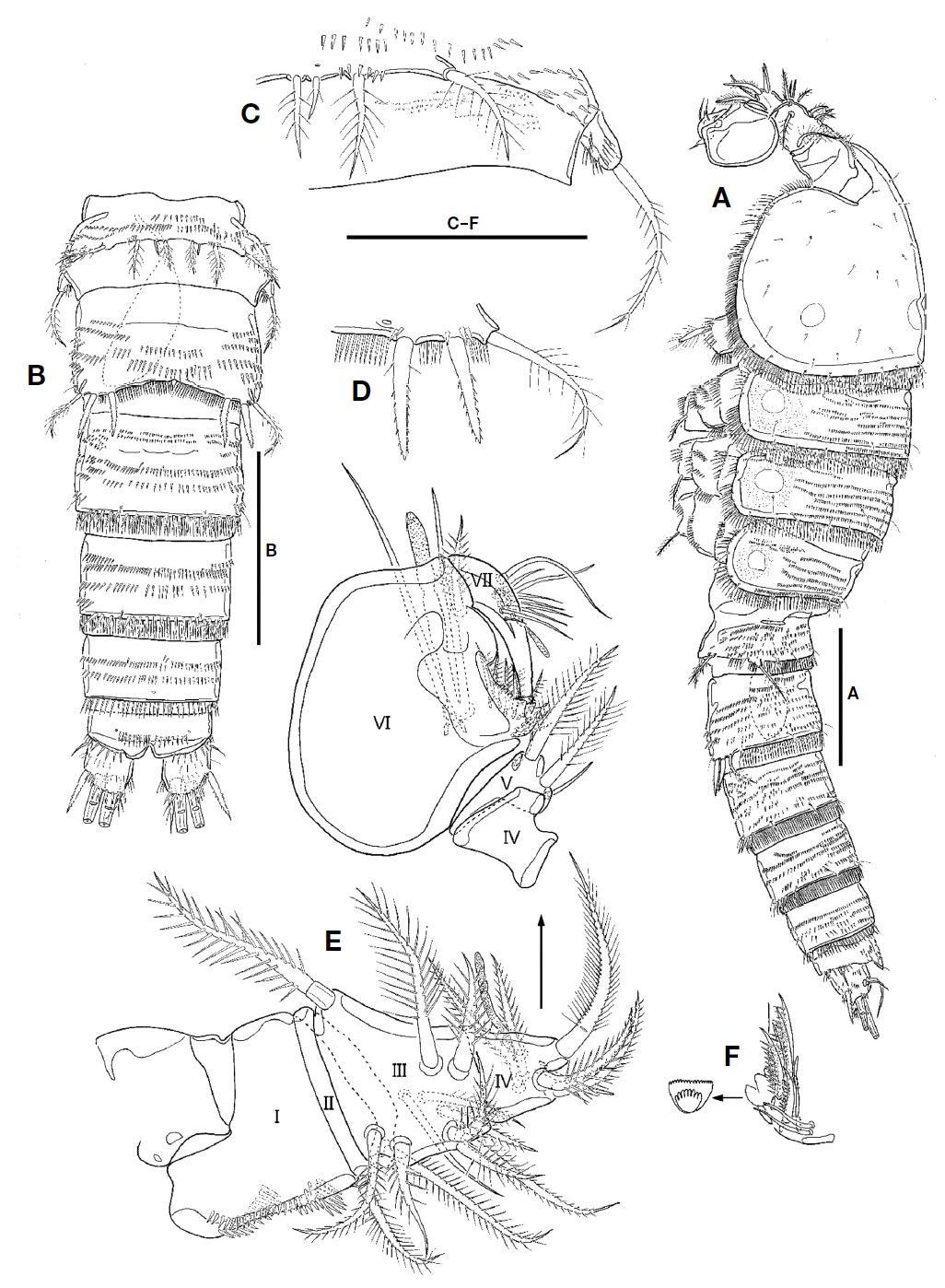
Description. Female: Body (Fig. 1A, B) fusiform tapering posteriorly, with distinct boundary between prosome and urosome; total length from 660.0 to 716.7 μm (mean 690 μm, n=4) including rostrum and caudal rami; surface of each somite armed with rows of minute spinules except for cephalothorax and caudal rami; posterior margin of each somite except for caudal rami armed with spinules; pleuron of each pedigerous prosomite armed with spinules. Rostrum (Fig. 2A) triangular in shape, fused to cephalothorax at its base, with 2 sensilla subapically; anterior tip blunt, with ventral protrusion bearing 2 sensilla. Cephalothorax (Fig. 1A, B) as long as wide in dorsal view, with row of spinules along posterior and anteroventral margins; surface covered with paired sensilla, with 3 nuchal organs posteriorly. P1-bearing somite incorporated into cephalothorax. Each pedigerous prosomite (Fig. 1A, B) with paired nuchal organs on lateral surface; surface with 2 pairs of sensilla except for P4-bearing somite; each posterior and anteroventral margins with 10, 8, and 2 pairs of sensilla, respectively. Urosome (Fig. 2B) slender than prosome, tapering posteriorly. P5-bearing somite (Fig. 1A, B) trapezoidal, with 2 pairs of sensilla on surface. Genital double-somite (Figs. 1A, B, 2B) slightly shorter than wide in dorsal view, fused ventrally but separated dorsolaterally by suture; genital somite with 2 pairs of sensilla on posterior margin and well-developed pleuron bearing rows of spinules; urosomite 3 narrower than genital somites, with 2 pairs of sensilla on posterior margin. Genital field (Fig. 2C) having common median genital slit without seta; single copulatory located posterior to genital slit and covered by cuticula process. Urosomite 4 with stout spinules on both posterolateral surfaces; posterior margin with 2 pairs of sensila dorsally and 1 pair of sensilla ventrally. Urosomite 5 slightly smaller than preceding one, with 2 pairs of sensilla on posterior margin. Anal somite (Figs. 1A, B, 2B) small; dorsal surface with 1 pair of sensilla; ventral surface with 1 row of spinules and 1 pair of sensilla; posterior margin armed with row of spinules ventrally; anal operculum weakly developed, semicircular, with 1 row of fine setules along posterior margin; anal opening ornamented with several rows of fine setules.
Caudal rami (Fig. 2D, E) as long as wide in ventral view, with 1 oblique row of setules on dorsomedial surface medially and several minute spinules on dorsal and lateral surfaces, and armed with 7 setae. Seta I small, located on lateral surface proximally. Seta II long, slender, and located on dorsal surface, near seta VII. Seta III spiniform, pinnate, longer than caudal ramus in length. Setae IV and V welldeveloped, pinnate distally; seta V 1.5 times as long as seta IV. Seta VI slender, small, slightly shorter than seta III and bearing setules. Seta VII articulated, slender, as long as seta III.
Antennule (Fig. 2F) short, blunt, 6-segmented; segment 1 largest, with 1 pinnate seta and 4 oblique rows of spinules; segments 2 with 1 minute bare and 6 pinnate setae; segment 3 with 4 pinnate setae; segment 4 smallest, with 1 pinnate seta and 1 peduncle having aesthetasc and long pinnate seta; segment 5 small, with 1 pinnate seta; segment 6 blunt, as long as two preceding segments combined, and bearing 1 plumose seta, 6 bare setae, 7 pinnate setae, and 1 aesthetasc. Each aesthetasc on segments 4 and 6 fused to pinnate seta at its base.
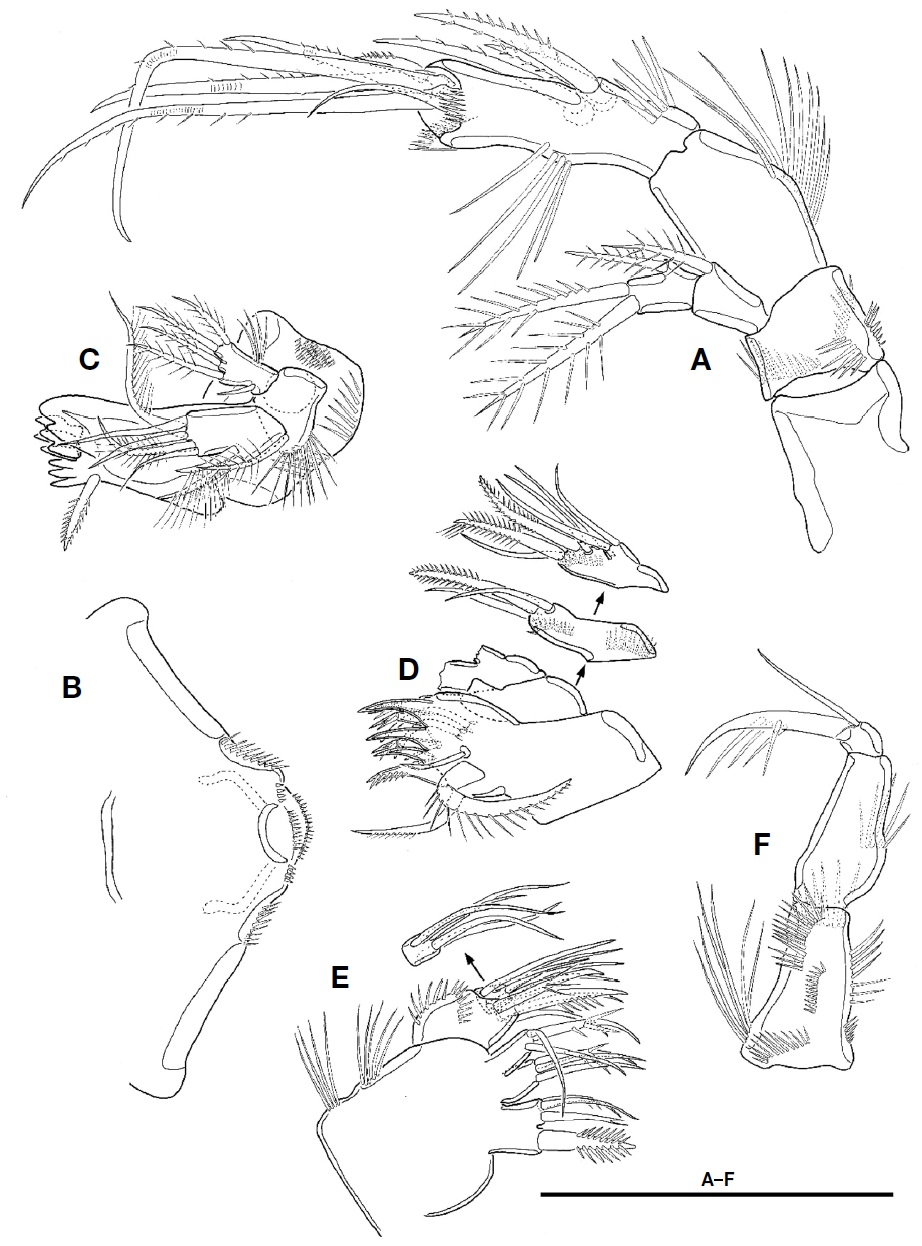
Antenna (Fig. 3A). Coxa without ornamentation. Basis small, shorter than coxa, with 4 rows of spinules. Exopod 2-segmented; proximal segment with 1 plumose seta at distal corner; distal segment slightly shorter than preceding one, with 1 lateral and 2 apical plumose setae. Endopod 2-segmented; proximal segment about 2 times as long as coxa, with 1 row of setules along abexopodal margin; distal segment longer and slender than preceding one, with 1 row of setules on inner margin, 1 row of spinules proximally and 3 pinnate spines on abexopodal margin, and 2 hyaline frills on distal margin; distal armature of distal segment composed of 1 small pinnate spine, 4 geniculate setae, and 1 small bare seta.
Labrum (Fig. 3B) well-developed, armed with paired spinular row along lateral margin distally, and 2 spinule rows and 1 paired spinule rows near distal margin.
Mandible (Fig. 3C). Gnathobase well-developed, with 1 tricuspid, 1 bicuspid, and 5 unicuspid teeth on cutting edge and 1 pinnate seta at distal corner; surface with 2 rows of setules and patch of small spinules. Palp biramus; basis broad, with 1 plumose seta and 1 row of spinules; exopod 1-segmented, small, with 1 bare and 4 plumose setae and 1 group of setules; endopod 1-segmented, elongate, with 2 uniplumose setae on lateral margin and 3 bare and 2 plumose setae on distal margin.
Maxillule (Fig. 3D). Praecoxal arthrite with 7 spines (3 naked, 3 bearing spinule, and 1 pinnate) and 4 setae (2 stout unipinnate, 1 small naked, and 1 small unipinnate); anterior surface with 2 juxtaposed setae. Coxa with 2 rows of spinules on anterior surface; endite with 1 stout pinnate and 2 bare setae. Basis with 1 row of spinules on anterior surface, 2 stout pinnate and 3 bare setae. Endopod small, 1-segmented, fused into basis at its base, with 2 apical setae. Exopod absent.
Maxilla (Fig. 3E). Syncoxa with 2 rows of setules along outer margin and 3 endites; proximal endite bilobate, with 1 stout pinnate seta on proximal lobate and 3 setae on distal lobate; both middle and distal endites each with 1 spinulose and 2 bare setae. Allobasis with 2 rows of spinules and bearing 2 spinulose and 2 bare setae. Endopod 1-segmented, small, with 5 elements.
Maxilliped (Fig. 3F) 3-segmented, subchelate. Syncoxa elongate with 6 rows of spinules and 1 group of long setules. Basis as long as coxa, with 1 row of setules near middle of outer margin. Endopod 1-segmented, small, with 1 long and 1 very small setae, and 1 long claw bearing accessory spinules.
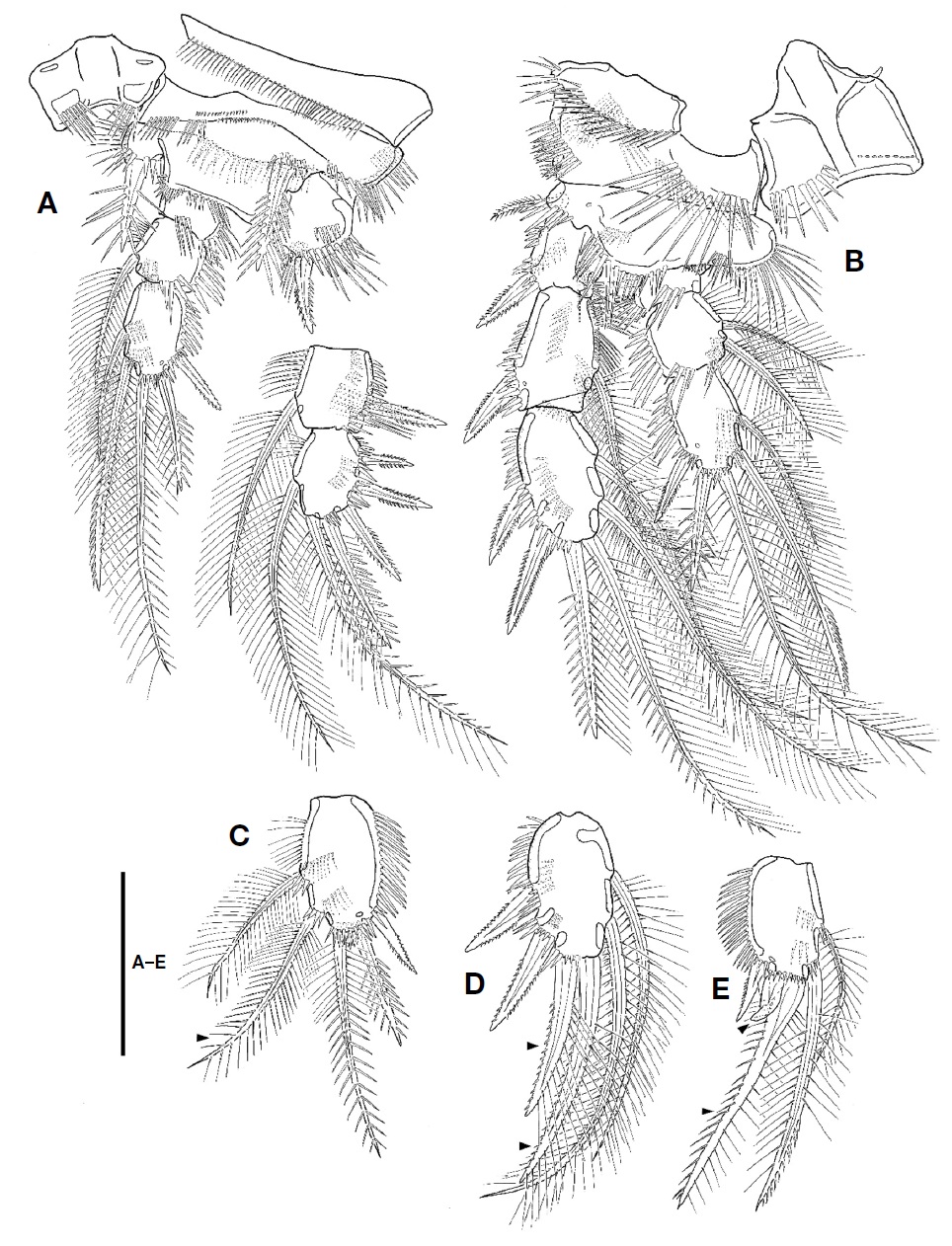
P1 (Fig. 4A). Intercoxal plate bilobate distally, with 1 row of spinules on each side. Praecoxal well-developed, with row of setules along distal margin. Coxa small, with 1 rows of small spinules on anterior surface and 3 rows of minute or moderate spinules along distal margin; outer margin with 2 rows of spinules posteriorly; distal margin with posteriorly 1 row of setules. Basis larger than coxa; inner spines spinulose, with spinules near its base; outer spine spinulose distally, with spinules near its base; inner margin with 1 row of setules; distal margin with 1 row of spinules. Exopod 3-segmented, longer than endopod, armed with several rows of spinules; exp-1 with 1 outer pinnate spine; exp-2 with 1 outer spine and 1 inner plumose seta; exp-3 with 3 outer spines, 2 apical setae, and 1 inner seta. Endopod 3-segmented, armed with several rows of spinules; enp-1 small, without inner seta; enp-2 with 1 inner plumose seta, 1 setule row on inner margin; enp-3 elongate, with 1 outer spine, 2 apical and 2 inner setae.
P2-P4 (Figs. 4B, 5A, B). Intercoxal plate armed with 2 spinule rows on anterior surface distally. Praecoxa smaller than that of P1, armed with rows of spinules distally. Coxa larger than that of P1; anterior surface with 1 long setule row and 1 spinule row; posterior surface with 2 or 3 spinular rows and 1 minute setule group; outer margin with 1 row of spinules. Basis with 1 plumose outer seta; anterior surface with 1 or 2 spinules distally and 1 pore; posterior surface with 1 or 3 spinule rows; inner margin with 1 row of long setules; distal margin armed with 2 or 3 spinule rows. Both rami 3-segmented, armed with several rows of spinules; exopod longer than endopod; exp-2 with 1 inner setule row and 1 anterior pore; exp-3 with anterior pore; enp-1 small, with row of spinules on anterior surface; enp-2 with 1 setule row on inner margin; enp-3 with 1 tube pore on anterior surface except for P4; distal seta on enp-3 serrate distally. Setal formula of P2-P4 as follows:
P5 (Fig. 5C) fused to somite. Outer peduncle located on lateral surface, armed with 2 rows of spinules, bearing 1 plumose apical seta. Exopod and baseoendopod fused, forming bilobate sling plate; outer lobe small with 1 plumose seta; inner lobe large, with 3 long plumose setae and 1 group of inner setules.
Male: Body shape and ornamentation similar to female (Fig. 6A); total length from 666.7 to 712.2 μm (mean 689.5, n=2) including rostrum and caudal rami; urosomites 2 and 3 separated each other (Fig. 6B).
Antennule (Fig. 6E, F) 7-segmented, blunt, and subchelate. Segment 1 with 1 plumose seta and 4 rows of spinules. Segment 2 very short, with 1 plumose seta. Segment 3 tapering distally, with 10 plumose or spinulose setae and 1 naked seta. Segment 4 with 5 plumose or spinulose setae, 1 naked seta, and 1 small aesthetasc. Segment 5 small, subtriangular in shape, with 2 plumose and 1 naked setae. Segment 6 large, swollen; palmar margin with 3 naked and 5 unipinnate setae, 3 small produces and 1 bub-like process; inner surface having 2 slender setae and 1 aesthetasc; distal corner with 1 spinulose seta; aesthetasc on inner surface fused to seta at its base; distal margin of bub-like process serrate in ventral view (Fig. 6F). Segment 7 hook-shaped, slender, with 9 bare setae and 1 aesthetasc.
P1 as female except for distal inner seta on enp-3, which plumose, not pinnate distally (Fig. 4C).
P2 as female except for exp-3 and enp-3. Two apical setae on exp-3 modified; outer margin of apical setae armed with very small spinules; inner seta on smaller that of female (Fig. 4D). Enp-3 with modified apical setae; outer seta very reduced, slightly curved outwardly; inner seta shorter than that of female, slightly swollen at its base (Fig. 4E).
P3 and P4 with modified apical seta on exp-3; outer margin of apical setae armed with very small spinules; inner apical seta on exp-3 smaller that of female (Fig. 5D, E).
P5 (Fig. 6C) incorporated into P5-bearing somite. Endopodal lobe weakly developed, small plate-like, and with 4 setae; second inner seta shortest. Outer peduncle separate from endopodal lobe, located on lateral surface, with long plumose apical seta.
P6 (Fig. 6D) symmetrical, represented by plate; each with 2 pinnate spines and 1 long plumose seta.
Remarks. The Korean specimens examined in the present study are herein attributed to
For male
Korean name:1*조막마디날래장수노벌레 (신칭)
>
Taxonomic notes and detailed morphological diversity of so-called Microarthridion littorale
(1) The female
(2)
(3) It is generally known that the second innermost seta on the female P5 is as long as the neighboring setae in
(4) Morphological differences between the previous records of
These discrepancies in detailed morphological features might be mainly caused by poor and brief descriptions as mentioned above. Among so-called