The promising energy device of the proton exchange membrane fuel cell offers an exceptional potential for a clean, efficient, and reliable power source [1]. In this system, noble metals such as Pt and Ru have been used as electro catalysts due to their high activity at low temperatures and resistance to the dissolution under acidic condition. However, since Pt is an expensive metal in limited supply, reducing the amount of its loading for electrode fabrication is one possibility [2]. Another way is to replace the expensive catalysts with non-Pt metals due to low cost, comparable performance and long-term stability, which has been a critical research topic for practical dissemination of fuel cell technology [3]. Many groups have tried to apply metal oxides (SnO2, TiO2, WOx, RuO2, and SiO2), metal nitrides, metal carbides and these hybrid supports but it was difficult to reach to the efficiency level of Pt based catalysts [3].
It has been found that some novel carbon nanomaterials with high surface areas, enough anchor sites, high electrical conductivities, and high oxidation resistance under strong oxidizing condition can be one of the ideal alternatives for PEMFC catalysts [3]. Especially, nitrogen (N) doped carbons such as N-doped bulk carbon, carbon nanotubes, and micro and mesoporous carbon materials have been recognized as a promising type of cathode catalyst due to the high ORR activity, good stability, easy synthesis process, inexpensiveness, and large variety of applications [4]. Among them, ordered mesoporous materials (OMC) have received enormous attention owing to their remarkable functional properties such as high surface area, regular frameworks, and tunable pore sizes with narrow pore size distribution, which makes it suitable for multiple potential applications in catalysis, adsorbents, sensor, electrode materials and energy storage devices [1-4]. These OMCs can be additionally doped by heteroatoms like nitrogen, which produce an excellent blend and can be very effective for largely enhancing their electro catalytic property [5-8].
The nitrogen doped OMCs can be synthesized by methods that are using mainly porous anodic alumina (PAA) or mesoporous silicas as templates and styrene, acetonitrile, aniline as precursors by a chemical vapor deposition approach. [9-11]. As the content and species of the nitrogen significantly affects to the ORR activity, efforts to enhance the nitrogen content in carbon structures is taken by employing new precursors with a high nitrogen content, such as ionic liquids (ILs) [12]. The resulting materials can possess high nitrogen content, local graphitic order, and excellent conductivities even at elevated carbonization temperatures. As the precursors are liquids at room temperature, they can be also easily incorporated into porous templates, avoiding any high pressure techniques. Porous nitrogen doped carbons generated from these precursors do indeed show a structural inversion of the template structure, thus enabling perfect nano-casting and structural control [13-17].
Due to improve the catalytic activity of nitrogen doped carbon, transition metals (Fe, Co etc.) are generally promoted to nitrogen complexes. The transition metal does not participate itself in the ORR. It serves to catalyze the formation of nitrogen doping structures in the carbon framework that are active toward the ORR by increasing the surface concentration of nitrogen groups and active sites [12, 18-23]. Furthermore, the presence of transition metals can enhance the electron donor property of the carbon matrix by catalyzing nitrogen doped graphitic carbon structure during the pyrolysis step [19]. This structure weakens the O-O bond via bonding between oxygen and nitrogen and/or the adjacent carbon atoms and facilitate the oxygen reduction reaction [24]. Apart from the disagreement on the ORR active sites, little, especially single transition metal promotion, has been currently known about the activity and stability of these non-precious cathode catalysts in the acidic operating conditions of PEMFCs [25, 26].
The aim of this work is to improve catalytic activity of the nitrogen doped mesoporous carbon catalysts, using dual transition metal promotion into the carbon structure. We investigate the different amount and species of the nitrogen functional groups in N-doped carbons which was carbonized from the precursor of ionic liquid (3-methyl- 1-butylpyridine dicyanamide) at various temperatures. The effect of the dual (Fe and Co) transition metal promotion was investigated by the evaluation of the ORR activities, and physical and chemical characterizations were carried out by XRD, XPS and TPO (temperature programmed oxidation) techniques.
The ionic liquid based ordered mesoporous carbons (ILCs) were synthesized by the ionic liquid and SBA-15. The ordered mesoporous silica of SBA-15 was used as a template [27], which was prepared by following the procedure by Mukaddes. C [28]. To investigate the structure of the carbons without transition metals, ionic liquid (3-methyl-1-butylpyridine dicyanamide, Sigma Aldrich) without metal were impregnated into the SBA-15 silica template. Subsequently, the ILC was carbonized at different temperatures of 500 ℃, 700 ℃ and 900 ℃ in N2 atmosphere for 3 hs. The ILCs were obtained by dissolving the silica templates in 1 M sodium hydroxide aqueous solution (Sigma Aldrich) at 90 ℃. Finally, ILC-500 ℃, ILC-700 ℃ and ILC-900 ℃ were produced.
Two different methods for the promotion of the transition metals to the ILC were introduced. (1)The ILC synthesized at the 900 ℃ which is named as ILC (further synthesized temperature will not noticed at the transition metal promoted sample name) was soaked in FeCl3 (Sigma Aldrich) and CoCl2 (Sigma Aldrich) aqueous solutions to prepare Fe and Co- promoted carbon catalysts. The aqueous suspensions were sonicated/stirred for 3 h, and solvent was then removed with evaporation at 80 ℃. In the second step, heat treatment was carried out in N2 at 1000 ℃ for 1 h to produce the catalyst. Fe and Fe+Co were deposited into the mesoporous doped carbons with metal amount of 1.5 wt%, which were optimized from our previous study [27]. The obtained catalyst were named as catalyst and denoted as ILC-Fe-HT (HT-heat treated at 1000 ℃) and ILC-Fe+Co-HT, respectively. (2)To reduce the synthesizing steps of transition metals promoted to the ILCs, the transition metals (Fe and Fe+Co 1.5% Wt%) were dissolved together with the ionic liquid and were sonicated/stirred for 3 hs, were impregnated into the SBA-15 silica template, and all the samples were carbonized at in N2 at 900 ℃ for 3 hs. The ILCs were obtained by dissolving the silica template in 1 M sodium hydroxide aqueous solution at 90 ℃. These samples were named by ILC with Fe and ILC with Fe+Co, respectively.
The TEM, XRD, XPS and temperature programmed oxidation (TPO) were used to characterization of synthesized ILC. The High resolution Hitachi H-800 system was used for TEM images obtaining. The Cu Kα-ray radiation (λ=1.5405 Å) utilized Rigaku-Miniflex diffractometer, which operating at 30 kV and 15 mA, was used to measurement of the XRD patterns. The XRD patterns were recorded between 2
2.2. Electrocatalytic Activity Evaluation
The glassy carbon electrodes embedded in Teflon polished with polishing cloth using alumina powder were used as working electrodes, for rotating disk electrode (RDE). A Gamry Framework potentiostat/galvanostat was utilized for the RDE measurements were carried out in a single compartment glass cell using a three electrode arrangement. All working electrodes were prepared according to: in the mixture of 1 ml ethanol and 80 μl 5% Nafion solution, 10 mg of sample was dispersed. It was then sonicated for 3 minutes and was for stirred 5 minutes. This procedure repeated five times. Then 2 μl of this suspension was dropped onto the glassy carbon electrode, dried at the room temperature. An RDE experiment was carried out at scan rate of 5 mV/s within the potential range of +1 V to −0.2 V in 0.5 M H2SO4. For oxygen saturation in the electrolyte solution, pure oxygen gas was applied. Commercial Pt/C catalyst (40 wt %) were used for comparison. The working electrodes were prepared in same way as those used in RDE were prepared. A platinum grid was used as counter electrode, and a double-junction Ag/AgCl 3M KCl electrode served as the reference electrode.
2.3. Fuel cell Polarization Measurements
The catalyst was ultrasonically blended with a Nafion solution (5 wt%, Alfa Aesar) and isopropyl alcohol for 30 min for preparation of cathode catalyst ink. The catalyst ink was sprayed onto a gas diffusion layer (GDL) (GDL-10bc) until the desired catalyst loading of 2 mg cm−2 was achieved. The weight percentages of catalyst and Nafion in the dried cathode layer were 50 and 50 wt%, respectively. The 40 wt% Pt/C was used anode catalyst, and the Pt loading was 0.5 mg cm−2. The MEA was hot pressed using a Nafion 212 membrane at 120℃ and 1 atm. for 3 mins. The MEA test was carried out in a single cell. Pure H2 humidified at 75℃ and pure O2 humidified at 75℃ were supplied to the anode and the cathode, respectively. The flow rates of H2 and O2 were 300 and 150 ml min−1, respectively. Polarization experiments were conducted at 75℃ using a fully automated test station without back pressure.
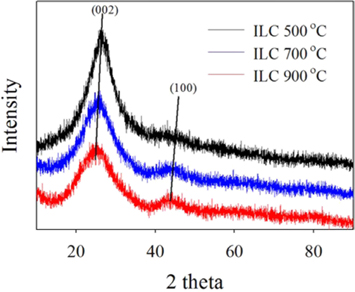
The synthesizing temperature dependencies of 500, 700 and 900 ℃, respectively, for ionic liquid mesoporous carbons (ILCs) were indicated by the XRD measurements of full range patterns, as in Fig. 1 indicate that ionic liquid heated to 500 ℃ already possess a well-developed graphitic stacking peak at 26.4°, which for higher-temperature samples is slightly shifted to smaller angles (24.6° for the product heated to 900 ℃), while its width stays constant or even slightly broadens with temperature. The more pronounced peak at 43.6°, however, points to the formation of a higher degree of interlayer condensation for the higher-temperature materials [13]. The results of the comparison of ILC-500 ℃, ILC-700 ℃ and ILC-900 ℃ revealed also that the synthesis at higher temperature induces less decrements in crystallinity with a higher graphitization. This trend can be connected with the characterization results from the XPS.
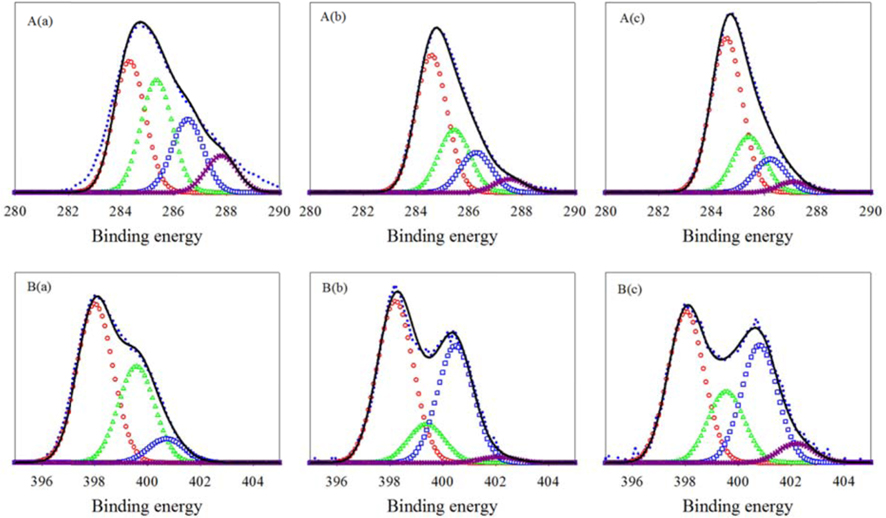
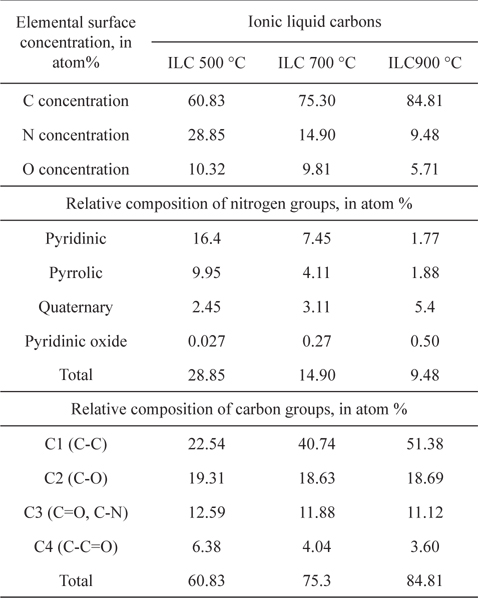
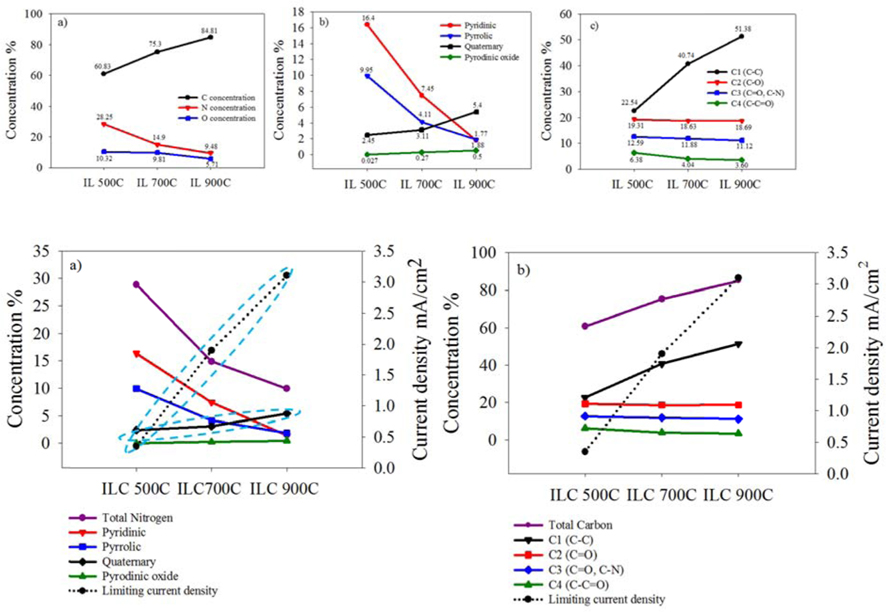
To investigate the chemical structures of the prepared samples, the C1s XPS spectra ranging from 282 to 290 eV for ILCs are shown in Fig. 2 and summarized Table 1. In Table 1, the total carbon concentrations were 60.83, 75.30 and 84.81% with a higher carbonization rate for ILC-500 ℃, ILC-700 ℃, and ILC- 900 ℃, respectively, which may provide better conductivity during the ORR. In general, there are several types of C groups in the synthesized ILCs by the appearance of several spectral peaks: C-C at 284.6 eV (C1), C-O at 285.5 eV (C2), C=O and C-N at 286.3 eV(C3) and C-C=O at 287.1 eV (C4) [27]. The peak intensities of the C-C (C1) species shows an increase depending on the temperature growth as shown in Fig. 2(a). On the other hand, the peak intensity of the COx species (C2-C4) is decreased as the synthesizing temperature is increased where the oxygen containing surface groups (COx) in the catalysts are not active sites [27]. The structure of ILCs, synthesized at various temperatures, were also analyzed by the characterization results from the N1s XPS, as can be seen in Fig. 2(b) and summarized deconvolution results in Table. 1. The N1s XPS of the ILCs is deconvoluted into four peaks pyridinic-N (398.6±0.3 eV), pyrrolic-N (400.5±0.3 eV) quaternary-N (401.3±.3 eV) and pyridinic oxide (403-405eV) [28]. While the total N concentrations in the ILCs decreases from 28.85 to 9.48 at.% as the synthesizing temperature increases, the change in the nitrogen species was obvious. When the synthesizing temperature of ILCs is increased, a decrease in the concentrations of the pyridinic-N and pyrrolic-N is detected. In contrast, the amount of quaternary-N is increased with increasing synthesizing temperature. Many electrocatalytic reactions show increased kinetics on carbon edge planes compared with basal planes. This increase in activity is attributed to the higher tendency of edge planes to chemisorb oxygen [29]. The pyridinic-N has a lone electron pair in the edge plane of the carbon matrix, the electron-donating ability of the N-containing carbon catalyst is increased by this electron pair, thus promoting the electrocatalysis of the oxygen reduction reaction. Liang et al. [30] proposed that the pyridinic-N may be not the definitive ORR active sites; but, according to their research results, the more catalytically active samples contain a higher percentage of pyridinic-N. Thus, it will weaken the O–O bond via the bonding between oxygen and nitrogen and/or the adjacent carbon atom, and facilitate the reduction of oxygen. Ikedaet al. [31] used the Car-Parrinello MD approach to model oxygen reduction on N-doped carbon layers and found that the O2 molecule is preferentially adsorbed associatively at C sites on graphene-like zigzag edges, if a quaternary-N is located nearby. The subsequent oxygen reduction proceeds mainly by the four-electron reaction pathway with a low free-energy barrier. Quaternary-N bonds to three carbon atoms in the plane of the carbon matrix. These type of nitrogen is the most stable species present in carbon when heated to 800 ℃ or higher temperatures [32]. There are two major viewpoints on the nature of the ORR active sites of these nitrogen-modified carbon-based catalysts. Based on previous research reports, the opinion was supported on the ORR activity of N-doped carbon, various nitrogen configurations are not all active sites for ORR. Furthermore, among the active sites, quaternary-N may be stable active sites while pyridinic-N is not that much stable for the acid condition as in the ORR [33].
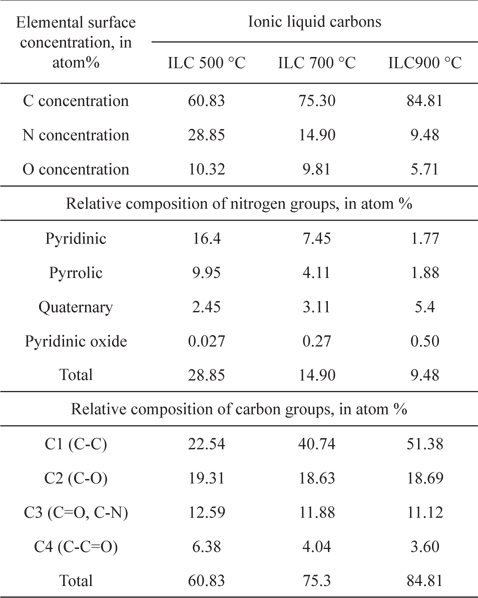
Elemental surface concentration and relative composition of nitrogen and carbon groups of ILC-500 ℃, ILC-700 ℃, and ILC-900 ℃.
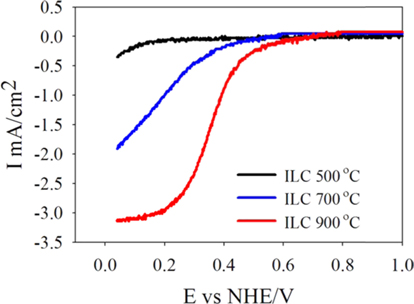
To evaluate these effects of the ILCs on the ORR activity, Fig. 3 shows the polarization curves for oxygen reduction on the ILC-500 ℃, ILC-700 ℃, and ILC-900 ℃ catalysts. All experiments were operated in O2 saturated 0.5 M H2SO4 at room temperature using a potential scan rate of 5 mV/s and rotation rate of 1600 rpm. In the case of synthesized ILCs at the various temperature, prepared catalysts reveal different onset potentials of 0.2, 0.54 and 0.69 V with different diffusion–limited currents of 0.35, 1.9 and 3.1 mA/cm2 for ILC-500 ℃, ILC-700 ℃ and ILC-900 ℃, respectively. Especially, the ILC-900 ℃ showed the highest ORR activity with a best onset potential and diffusion–limited current. This result came from the physical and electrochemical characterizations that suggest the total nitrogen amount and all nitrogen configurations are not the active sites for ORR, though quaternary nitrogen may be the active sites for the increase in electrochemical activity and conductivity by the graphitic structure [33]. It can be concluded that the ORR activity is related to the content of quaternary nitrogen, which depends on the synthesis temperature.
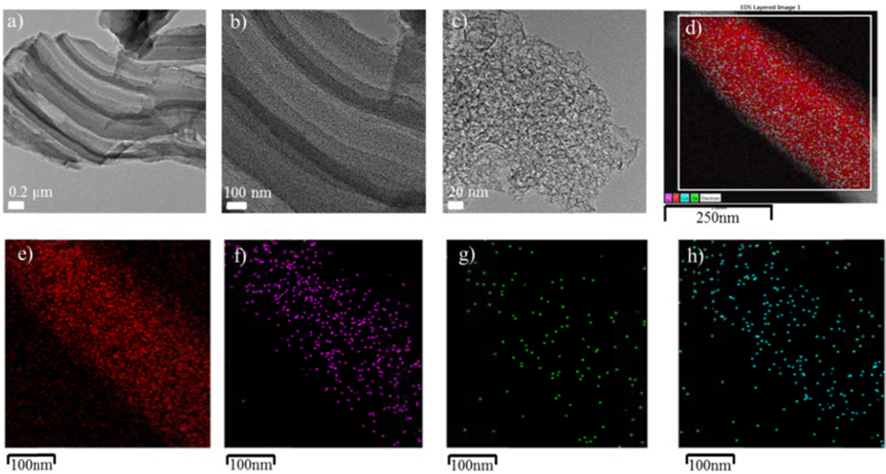
For the preparation of non-noble metal catalysts for the ORR, dual transition metal system of Fe and Co were inserted into the ILCs structure to evaluate the positive effect on the ORR. Yeager [34] and Wiesener [35] have suggested that the transition metals may not act as an active site for oxygen reduction but rather serve primarily to facilitate the stable incorporation of N into the graphitic carbon during high-temperature pyrolysis of metal-nitrogen complexes. This means that high temperature pyrolysis in the presence of transition metals yields a carbonaceous layer with substantial nitrogen groups that are catalytically active for oxygen reduction. In author’s previous study [27], non-noble metal mesoporous carbon catalysts were prepared through transition metal promotion with an optimal metal loading of 1.5 wt % to carbon, which motivated the preparation of ILCs with Fe, Fe and Co mixture (Fe+Co) loading of 1.5 wt % in this study. For the first method, further ILC-900 ℃ was chosen for the initial carbon material and transition metal promotion was transformed to second step heat treatment at 1000 ℃ (ILC 900 ℃ 1.5% Fe, (Fe+Co)-1000 ℃). To reduce the preparation methods of transition metal promotion, the second method approached one step synthesizing of ionic liquid and addition of metal salts. The synthesizing temperature was the same as above with 900 ℃. A shown in Fig. 4, the TEM images show that the structure of prepared transition metals promoted ILCs is highly porous, tight arranged and well ordered. Furthermore, from the EDS analysis, the amount of the Fe and Co were confimed as we inserted in the carbon structure and these nano particles are also well-dispersed in the ILCs structure.
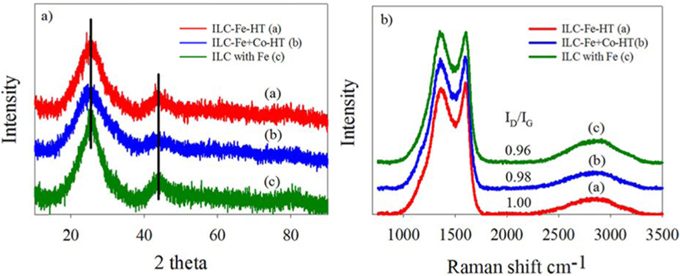
Fig. 5 shows the XRD and Raman spectroscopy results of ILC-Fe-HT, ILC-Fe+Co-HT and ILC with Fe to investigate the structural change. From all the XRD patterns in Fig. 5a, the graphite peaks in the (002) plane 2θ=24.8 plane are distinguishably appeared, confirmed a graphite structure. After the transition metal promotion and heat treatment at 1000 ℃, the peak intensities of all of the ILCs significantly increased. This result revealed that the transition metals (Fe and Co) and heat treatment induce an enlargement in crystallinity for N-doped ILCs. Specially, XRD peaks shifted to lower angles and the peak intensity is increased for the ILC with Fe compare with ILC-Fe-HT. This may be due to the greater graphitization through the one step synthesizing of ionic liquid and metal salts. For the case of the dual transition metal doping, a similar tendency was shown for the ILC- Fe+Co-HT compare with ILC-Fe-HT, which also explained the effect of the Fe and Co mixture promoting to the graphitization of the ILCs. When the graphitic structure increases, graphitic-N species of quaternary-N species increase which is related to the conductivity [18]. It could be expected that higher amount of quaternary-N species in the ILC-Fe+Co-HT and ILC with Fe can be observed than the ILC-Fe-HT due to two reasons of one step synthesis or dual metal doping.
This trend is also observed in the Raman spectroscopy for all sp2-carbons. As can be seen in Fig. 4(b), two conspicuous peaks emerged at approximately 1600 cm−1 of the G-band and 1360 cm−1 of the D-band. The G-band peak resulted from the E2g vibrational mode in the D 46h symmetry group of the graphite crystal planes while the D band originated from lattice distortion in the sp2-hybridized carbon and becomes active due to the presence of various defects (
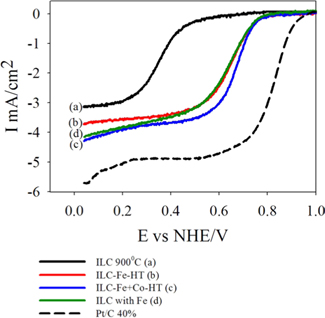
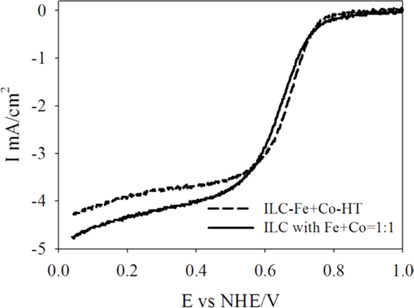
Fig. 6 shows the catalytic activities of the prepared catalysts in oxygen saturated 0.5 M H2SO4 solution at room temperature using a potential scan rate of 5 mV/s and a rotation rate of 1600 rpm. In the cases of 1.5% Fe, and Fe+Co deposited catalysts, ORR activities are largely increased compare to the catalysts without transition metals. After the promotion of transition metals, for numerical examinations from ORRs, the onset potentials were all increased from 0.69 to 0.9, 0.92 and 0.9 V vs normal hydrogen electrode (NHE) and the diffusion–limited currents (mA/cm2) were 3.73, 4.31 and 4.15 mA/cm2 for ILC-Fe-HT, ILC-Fe+Co-HT and ILC with Fe, respectively. Hence, it was considered that the transition metals have a positive effect on the oxygen reduction of the nitrogen doped carbons from ILCs. By comparing the modification of the transition metal promotion and promotion methods in Fig S4, onset potentials were increased from 0.92 to 0.99 V (vs NHE) and the diffusion–limited currents (mA/cm2) were increased from 4.31 to 4.75 mA/cm2 for ILC-Fe+Co-HT and ILC with Fe+Co=1:1, respectively. Hence, it was found that the dual metal promotion system and one step synthesis of the nitrogen doped carbons from ILCs shows positive effects on the ORR activity than only Fe promotion and heat treatment at 1000 ℃. Based on the XRD and Raman analysis, it can be seen that the structural change configured by the metals promotion to ILC catalyst, plays a key role in the ORR activity because the ORR activity depends on graphitic carbon structure [4, 27]. This shows that the best way of transition metal promotion on the nitrogen doped mesoporous carbon from ILCs is finally the one step synthesis with Fe+Co metal mixture.
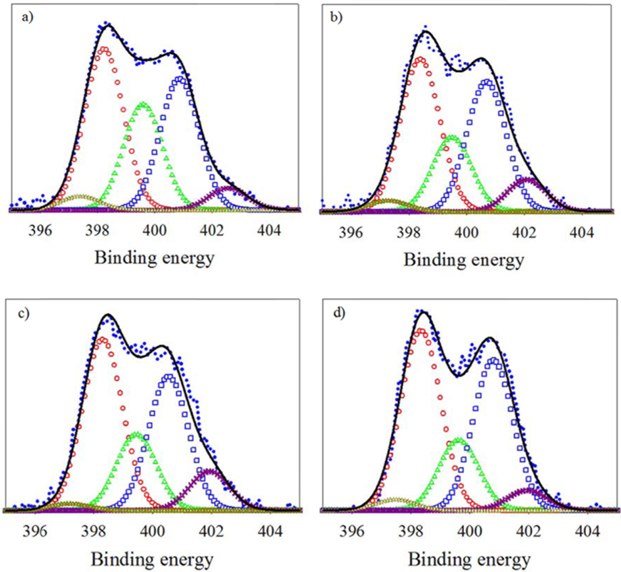
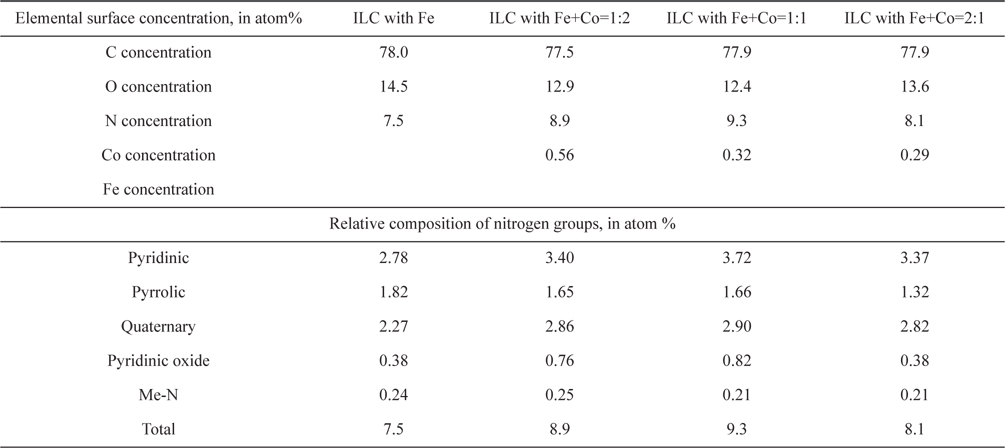
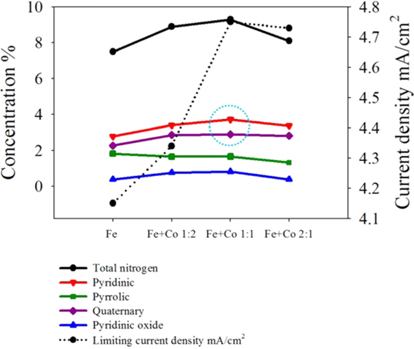
To evaluate the effect of Fe and Co ratio on the ORR, ILC catalysts with various Fe+Co ratios (1:2, 1:1, 2:1) were synthesized. There were named ILC with Fe+Co-1:2, ILC with Fe+Co-1:1 and ILC with Fe+Co-2:1. XPS analysis was performed to investigate the changes in the chemical structure of the nitrogen doped mesoporous carbons with transition metals Fe+Co mixture. The results are shown in Fig. 7 and Table 2. The deconvoluted results in the Table 2 indicate that the total N content of catalysts was the highest for the ILC with Fe+Co 1:1. Compared to the N 1s spectra of the ILC with Fe, ILC with Fe+Co 1:2, ILC with Fe+Co 1:1 and ILC with Fe+Co 2:1, the peak intensity and amounts of pyridinic-N and quaternary-N in N 1 s spectra were increased for all samples when the portion of the quaternary-N is relatively higher when the Fe and Co ratio is 1:1. Meanwhile, two nitrogen species of pyridinic-N and quaternary-N are both considered as active sites for the electrochemical reactions. Pyridinic-N contributes one p-electron to the aromatic π system and has a lone electron pair in the plane of the carbon matrix and at the carbon matrix plane, quaternary-N bonds to three carbon atoms. Okada et al [36] followed the approaching model of oxygen reduction reaction on the N-doped carbon layers, modified by the Car-Parrinello MD, which is founded like at the carbon sites on graphene-like zigzag edges if a quaternary-N is located nearby the O2 molecule is preferentially adsorbed associatively. Therefore, the quaternary-N is also an important species for the subsequent oxygen reduction, which mainly takes the four-electron reaction pathway, with a low free-energy barrier. After the transition metal promotion, the Me–N peaks are generated as shown in Fig. 8. A small peak at 394.7 eV is assignable to the Me-N related the bond (Me-N) [37]. The Me-N type N comprises only a very small portion (0.21-0.25 at%) of total surface N species (Table. 2), so it is difficult to evaluate the effect of Me-N in this case. In particular, transition metal, ratio (Fe+Co 1:1) positively contributes to the formation of graphitic-N, which is known to be an active site for the ORR.
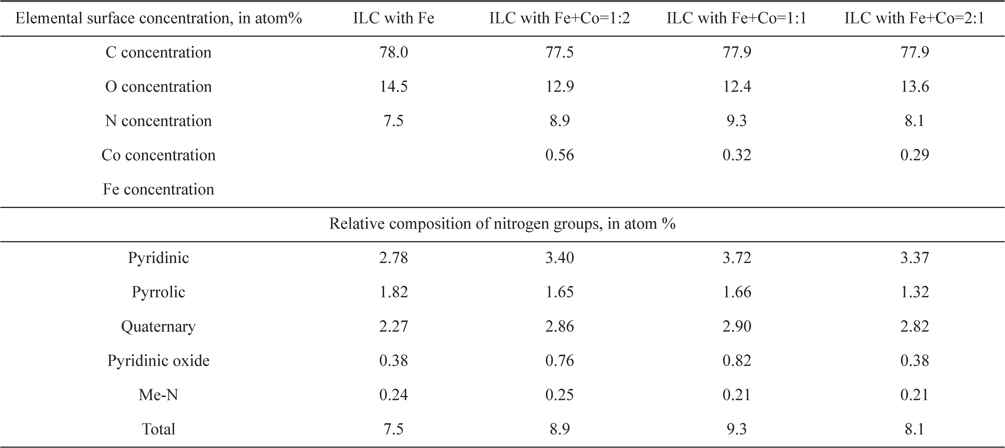
Elemental surface concentration and relative composition of nitrogen groups of carbon catalyst of ILC with transition metals. Metal ratio was Fe+Co= 1:2, 1:1 and 2:1.
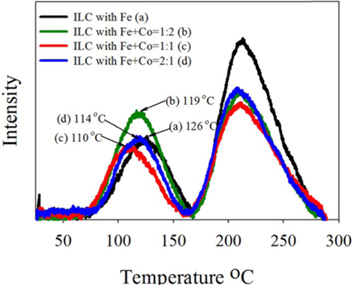
The oxygen reduction ability on the prepared mesoporous doped carbons based on the structural difference was possible to investigate through the O2-TPD experiments. To evaluate the oxygen adsorption property, the chemisorbed oxygen was desorbed at the argon carrier gas stream. It can be understood that desorption amount of adsorbed oxygen is low when the intensity of first peak is small. On the other hand, the reduced oxygen amount is high when the peak intensity is high. As shown in Fig. 8 of the O2-TPD, the peak intensities decrease in the case of ILC with Fe+Co 1:1-900 ℃ than the Fe, Fe+Co 1:2 and Fe+Co 2:1, indicating a decrease in the desorption amount of oxygen. This indicates that reduction adsorbed oxygen was greater in ILC with Fe+Co-1:1 than the others. These results show the same tendency with the increase in portion of the pyridinic N with a relatively high graphitization of the nitrogen doped mesoporous carbon, which was observed in the XPS data. From the O2-TPD results, it was also seen that the desorption peaks exposed to lower temperature is understood as a higher activity and easy reacted. In the case of ILC with Fe+Co-1:1, desorption peak is exposed at lower temperature of 110 ℃. Moreover, the second peak of O2-TPD at 200-250 ℃ is generally related to the decomposition of carboxylic acid [38]. The low intensity of the second peak of ILC with Fe+Co 1:1-900 ℃ shows that the carbons were less oxidized during the TPO test, which can be interpreted to that less oxidized carbons are more stable. As a result, the transition metal promotion ratio Fe+Co 1:1 in the nitrogen doped mesoporous carbon form ILCs shows higher activity than Fe, Fe+Co 1:2 and Fe+Co 2:1.
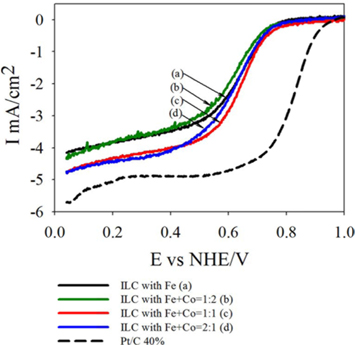
To evaluate the effect of doping transition metals ratio Fe+Co (1:2, 1:1 and 2:1 ) on the ORR, Fig. 9 shows the polarization curves in O2 saturated 0.5 M H2SO4 at room temperature using a potential scan rate of 5 mV/s and rotation rate of 1600 rpm. In the case of nitrogen doped mesoporous carbon catalysts with various ratios of the transition metals, all carbons reveals an onset potential of about 0.9V with a different diffusion–limited current of about (mA/cm2) 4.15, 4.34 and 4.73 for ILC with Fe, ILC with Fe+Co-1:2, and ILC with Fe+Co-2:1 respectively. Especially, ILC with Fe+Co-1:1 showed the highest ORR activity with onset potential 0.99 V and diffusion –limited current of about 4.75mA/cm2. These results were quite related to the O2-TPD results and provide a valuable information that the N doped mesoporous carbon form ionic liquid with Fe+Co 1:1 has a relatively proper chemical structure for the ORR based on other characterization tools.
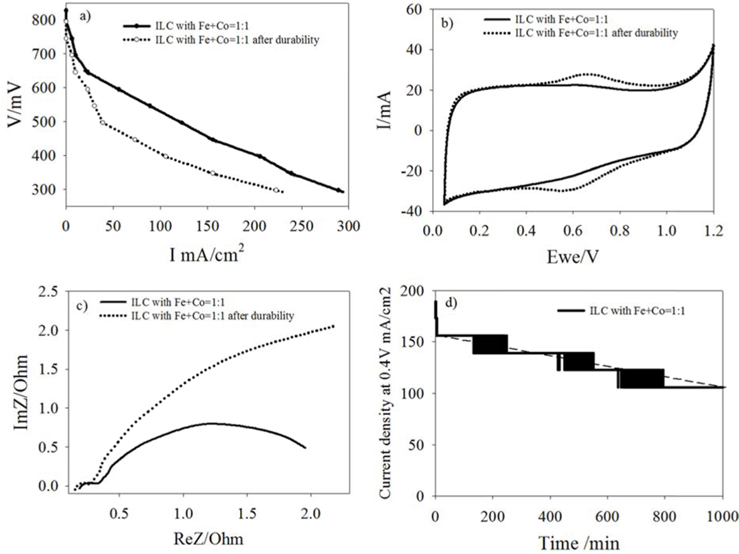
Figure. S1 shows the single cell test results of PEMFCs prepared with the ILC with Fe+Co-1:1 cathode catalyst. The cathode catalyst loading was maintained with relatively low amount of 2 mg/cm2 compare to other groups. The experiments were performed without a back pressure. Ohmic potential drop is not compensated in the measurements. The open circuit potential and current density of the ILC with Fe+Co-1:1 catalyst were 0.82 V and 220 mA/cm2 at 0.4 V, respectively. The relationship between the nitrogen species in the N1s region and the loss of catalytic activity is evaluated in a short term durability test. After the short term durability test in the case of ILC with Fe+Co-1:1, the open set voltage and current density was decreased and the catalyst resistance is increased. This loss of activity appears to be directly related to the protonation and oxidation of the pyridinic-N and formation of an inactive site (inactive metal particles on the carbon surface) [4, 27]. However, when the catalyst is compared with the CNx catalyst that was synthesized from PANI at 900 ℃ with single transition metal (Fe) promoted at the 1000 ℃, as our previous study [27], the catalyst activity loss is lower than CNx. After ten hours, the current density loss was 2.7 mA per hour for the prepared catalyst and 17 mA per hour for the CNx. The relationship between the N species and short term durability behavior corroborate the indication of that the quaternary-N is a stable active site. In the case of ILC with Fe+Co-1:1, the portion of the quaternary –N specie was higher (2.9 at%) than the CNx (1.87 at%). It can be concluded that the dual transition metal promoted ILC can be a promising catalyst in PEMFC.
Nitrogen doped mesoporous carbon catalysts with dual transition metal promotions were synthesized by carbonization from ionic liquid with silica hard templates for various temperatures. The influences of the synthesizing temperatures of ILC were explored by the electrochemical measurement and XPS analysis on the ORR activity. When the synthesizing temperature is increased (500℃ to 900℃), ORR activity of ILC is increased and exhibited good ORR activity at the 900 ℃ in the acid condition. In the case of N species ILC, total nitrogen amount and pyrrolicN species is decreased because these nitrogen species are unstable at high temperature and may change to other configuration of the quaternary-N species. The physical and electrochemical characterization results suggest that quaternary–N may be the active sites for the ORR. We also found that the dual transition metal (Fe and Co) promotion is a better system than the single transition metal promotion on the N doped carbon catalyst for the ORR. Larger amount of pyridinic-N and quaternary-N species were configured, which is favorable for improving the ORR activity by providing active sites and an effective electron path during the ORR. At the transition metals (Fe and Co) ratio of 1:1, this catalyst shows the highest ORR activity from the higher amount of pyridinic-N and quaternary-N. In comparison, ILC with Fe+Co-1:1 with PANI carbon catalyst, ILC catalyst exhibited to be more stable than the PANI catalyst at the PEMFC. Therefore, the ionic liquid may be a favorable source of both carbon and nitrogen in the nitrogen doped carbon catalyst for the ORR and especially, the dual transition metal promotion can be a encouraging method to produce a good non-Pt catalyst for PEMFC operation.