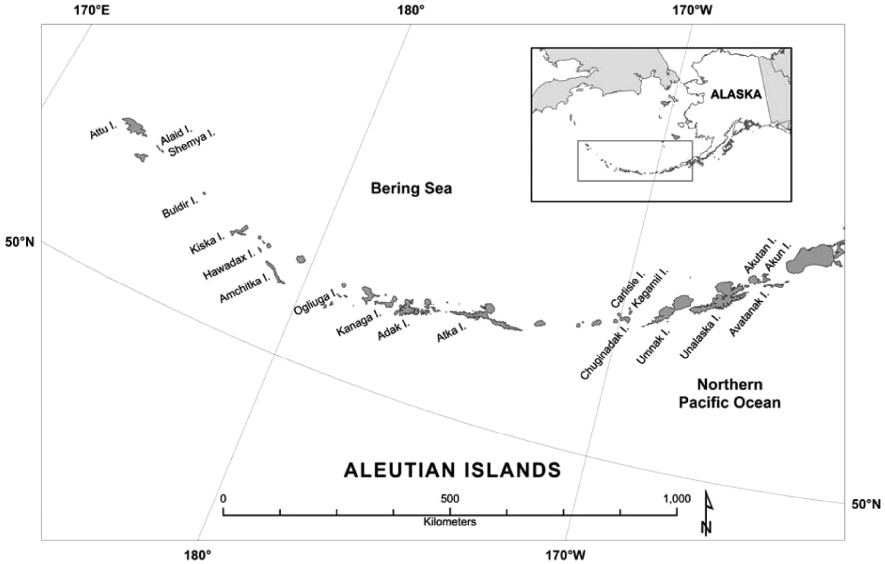
The Aleutian Islands, an archipelago of more than 300 islands, stretch more than 1,600 km from the western tip of the Alaska Peninsula at 163° W latitude (Unimak Island) to beyond the International Dateline at 172° E longitude (Attu Island) and span over 425 km of latitude (between 51 and 55° N). This remote archipelago is uniquely situated between the American and Asian continents and between two major bodies of water, the North Pacific Ocean and the Bering Sea (Fig. 1). The easternmost islands are situated on continental crust, whereas those west of Samalga Pass are distinctly oceanic. The islands are grouped in five major clusters, which are separated by increasingly wider and deeper passages from east to west (Stabeno et al. 2005). Ocean mixing and transport along the archipelago are driven by upwelling, tidal action through passes, and three major currents: the Alaska Coastal Current and the Alaska Stream flow westward on the Pacific side, and the Aleutian North Slope Current flows eastward on the Bering side (Ladd et al. 2005). Sea surface temperatures range from 5 to 10℃ in summer and drop to near 0 in winter (Rodionov et al. 2005).
The shores of the Aleutian archipelago offer some of the most exposed habitats of any rocky coastline on earth and experience a cool, wet and windy marine climate. Extreme winter storms are fueled by the notorious low-pressure area known as the Aleutian Low, and moisture-laden fog often blankets the coast in summer (Rodionov et al. 2005). Coastal areas are generally characterized by high cliffs with rock ramps (≥100 m wide) or steep bedrock, boulder, and cobble beaches. Substrates range from basaltic lava flows to tuffs, breccia, conglomerates, and sandstone (Gard 1977). Oceanic ground swells constantly carve shorelines, forming wave-cut channels, rounding boulders and cobble, eroding sea cliffs, and creating extensive spray zones. In addition to wind, weather and waves, the islands are also home to 37 extant volcanoes, and eruptions impacting coastal habitats are common (Jewett and Drew 2014). The archipelago’s vast and complex physical setting creates highly variable and isolating conditions between island groups and even neighboring islands. Endemism can be expected along this extensive but narrow chain of islands (Kawai et al. 2008, Jewett and Clark 2011).
Despite this forbidding environment, marine macroalgae thrive in the Aleutian littoral zone, including members of the Bangiales (Lebednik and Palmisano 1977). In fact, the wide spray zone of the upper intertidal is ideal habitat for many species of Bangiales, which favor cool waters with frequent disturbances that not only provide cleared substrate for colonization but also challenge the tenacity of invertebrate herbivores. Species of Bangiales often form a distinct band visible from offshore and can be an abiding feature of the Aleutian littoral zone. Although species of Bangiales are commonly found throughout the Aleutian Islands, their identity and distribution have been poorly documented because of the lack of collection due to remoteness and the challenging environment of the archipelago and the application of appropriate methods of determination. Wynne (1972) published the only study that focused on foliose Bangiales in this region, but his efforts were limited to Amchitka Island. Conway et al. (1975) included a number of records of Bangiales from the Aleutian Islands in their work. Both of these studies, however, were done at a time when morphology was still the main method for seaweed identification. Today, DNA sequencing is essential for identification of species such as foliose Bangiales, which have few morphological characters to distinguish them (Sutherland et al. 2011). Lindstrom (2008) and Lindstrom et al. (2015) included records of foliose Bangiales from the Aleutian Islands, but that region was not the focus of their studies.
We have now sequenced the
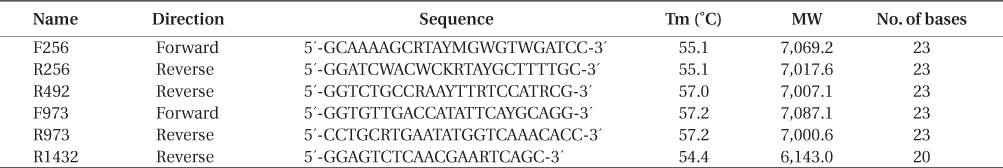
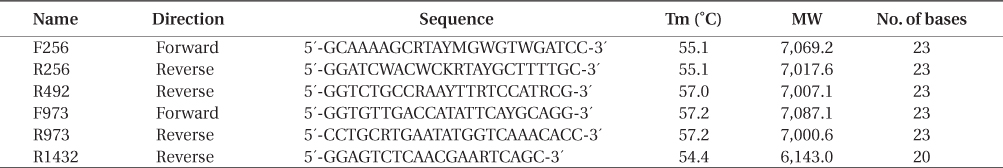
Specimens were collected in the Aleutian Islands in 1990, 1992, 1993, 1999, and 2000 by D. A. Guthrie, in 2004, 2005, and 2008 by S.C. Lindstrom, and in 2006 and 2007 by M. R. Lindeberg. Some of these were included in earlier publications (Lindstrom 2008, Lindstrom et al. 2015); the rest are listed in Appendix 1. Specimens were pressed fresh on herbarium paper with a small portion of the 2004-2008 specimens desiccated in silica gel for later extraction of DNA. These herbarium vouchers are deposited in the UBC herbarium. DNA extraction, amplification and sequencing followed Lindstrom (2008) with the replacement of the forward primer F57 with KitoF1 (5′-TGTCTCAATCCGTAGAATCA-3′) and the use of Bangiales-specific primers for material not desiccated in silica gel or otherwise difficult to amplify (Table 1).
[Table 1.] Bangiales-specific rbcL primers used in this study
Bangiales-specific rbcL primers used in this study
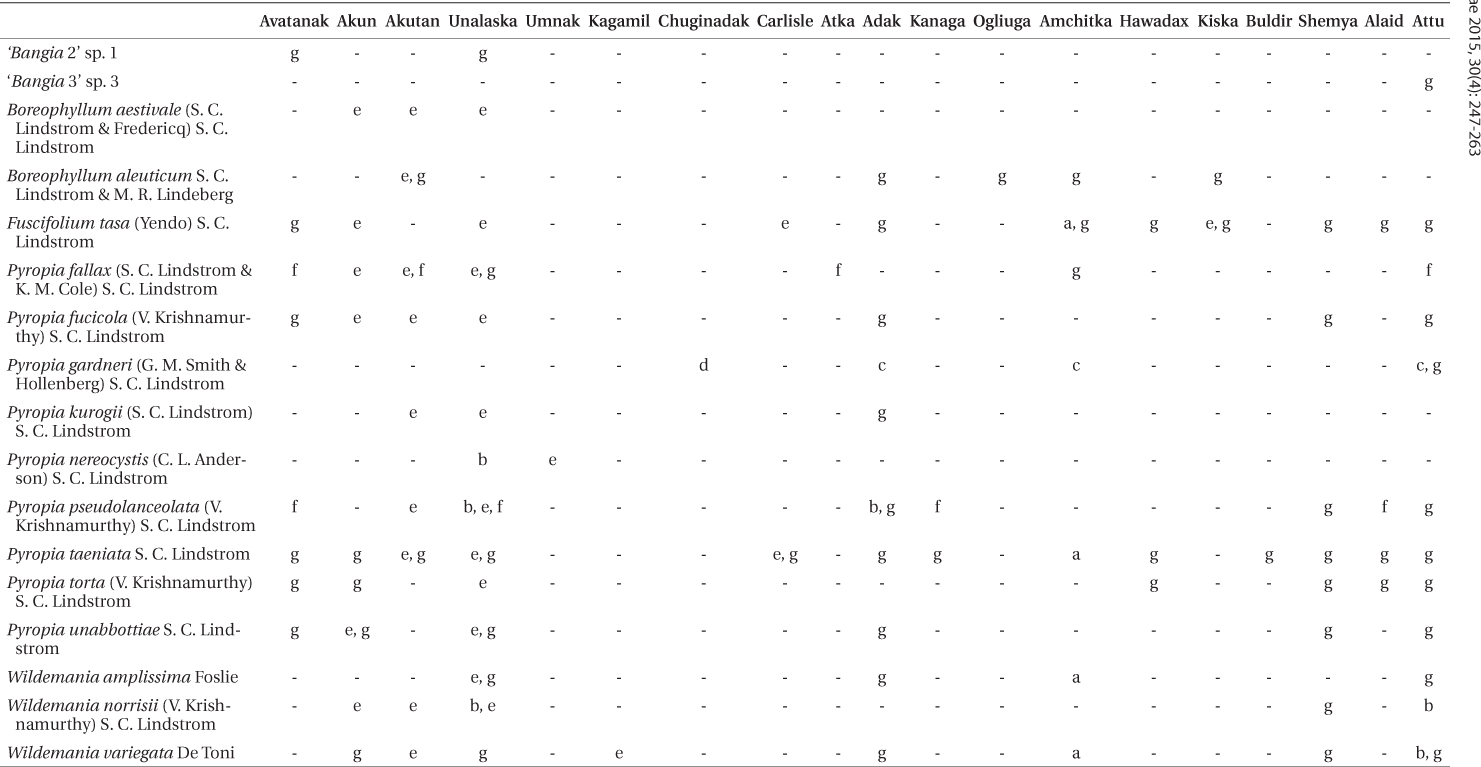
We identified 17 species of Bangiales in our Aleutian collections based on DNA sequencing (Table 2). Twelve of these have been described previously. Two
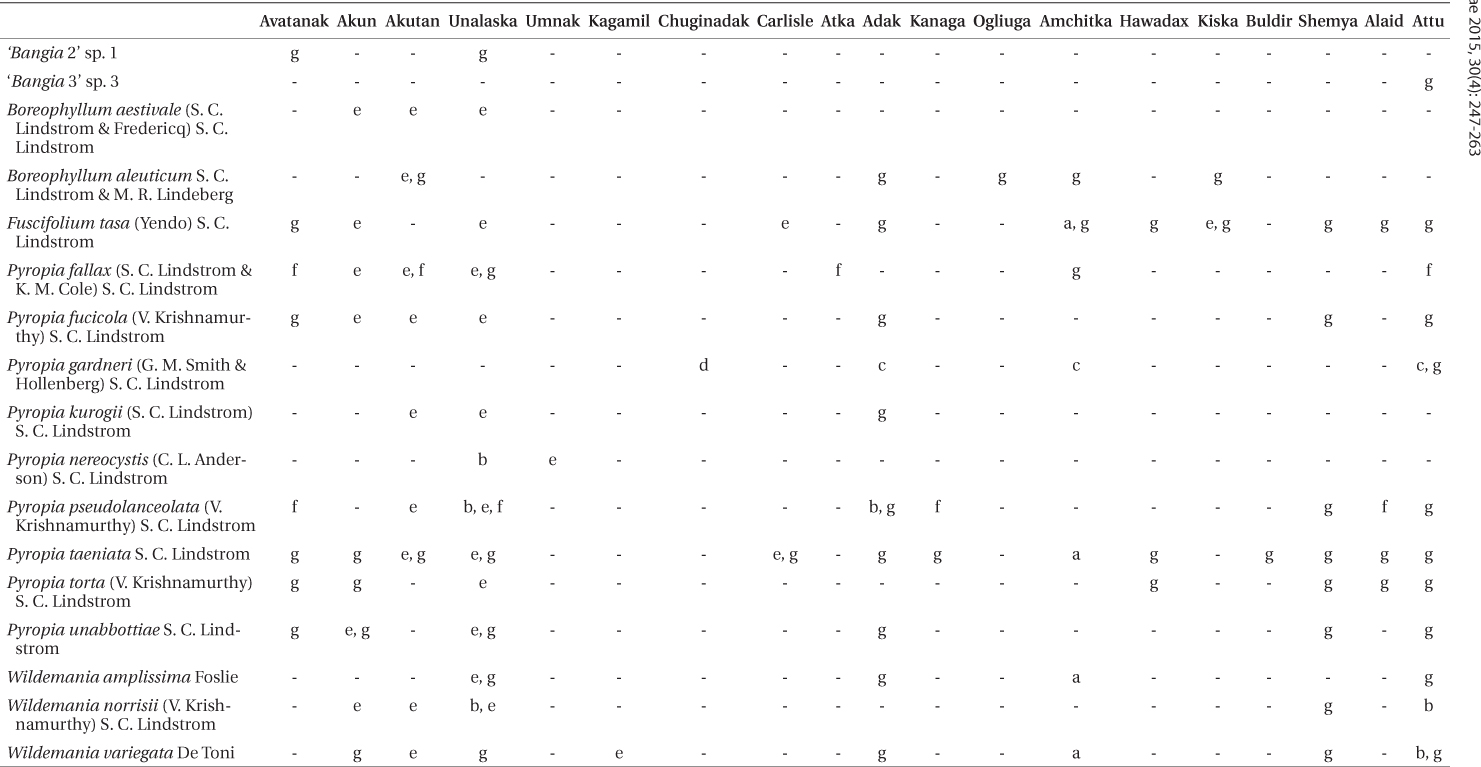
Verified Aleutian Islands distributions of species from this and previous studies (locations listed from east to west)
>
Boreophyllum aleuticum S. C. Lindstrom & M. R. Lindeberg sp. nov. (
This species was identified as Unknown #4 in Lindstrom (2008).
Description. Thalli to 1 m in length and width although usually much smaller, broadly ovate, obovate, or nearly orbiculate, often lacerated or even lobed, with margin very slightly but deeply ruffled. Base cuneate to umbilicate. Monostromatic, with a single chloroplast per cell. Dark red, brown to nearly black, oily-looking when alive; dusky purple when dried. Adhering well to paper. Vegetative thalli 50-85 μm thick. Male and female reproductive cells on separate plants. Spermatangia in irregular marginal sections of thalli, mostly distal, occurring according to the Hus formula 2-4/a, 2-4/b, 8-16/c (Hus 1902); spermatangial thalli 75-100 μm thick. Zygotosporangia in nearly inconspicuous reddish marginal sections, occurring according to the Hus formula 1-2/a, 1-2/b, 2-4/c; zygotosporangial thalli ~75 μm thick.
Holotype. UBC A90644, Haycock Rock, Kiska Island, low intertidal, Jun 30, 2007, Leg. M. R. Lindeberg ALEUT07_119. GenBank KT936157. The holotype consists of two thalli, one spermatangial, the other zygotosporangial.
Isotype. ALEUT07_118 in UBC (A90643) consists of a single thallus, which is spermatangial.
Etymology. Thus far, this species has been collected only in the Aleutian Islands, hence its name.
Habitat. On cobble or larger rock in the mid to low intertidal zone.
Phenology. Specimens have been collected only in May, June, July, and August (the only months when Aleutian sites were visited). Spermantangia were observed in early June collections as well as later but zygotosporangia not until late June.
Known distribution. Thus far known only from the Aleutian Islands: Akutan, Adak. Ogliuga, Amchitka, and Kiska (Table 2,
Comments. This species clearly belongs in the genus
>
Boreophyllum ambiguum S. C. Lindstrom sp. nov. (
Description. Thalli to at least 50 cm in length and width although usually much smaller, broadly ovate, obovate, or nearly orbiculate, often lacerated or even lobed, with margin moderately ruffled, particularly proximally. Base umbilicate. Monostromatic, with a single chloroplast per cell. Dusky purplish rose when dried. Mostly adhering well to paper. Male and female reproductive cells on the same thalli but on separate “halves,” separated by a very irregular, often inconspicuous vertical line. Spermatangia in more or less solid smears across the distal end of thallus, occurring according to the Hus formula 2-4/a, 2-4/b, 8/c; spermatangial thalli 52-65 μm thick. Zygotosporangia in mottled, very slightly rosaceous smears, occurring according to the Hus formula 1-2/a, 2/b, 4/c, with clearly visible cell walls separating packets; zygotosporangial thalli 55-85 μm thick.
Holotype. UBC A90188, head of Northeast Harbor, Sanak Island, Alaska, lower mid intertidal, on large boulder, Aug 31, 2012, Leg. S. C. Lindstrom 15128. GenBank KT936164.
Isotypes. SCL 15130 in UBC (A90189). GenBank KT93 6165.
Etymology. The species epithet derives from the difficulty in differentiating this species from the other species of
Habitat. Mid intertidal boulder, cobble, and pebble.
Phenology. Specimens have been collected in July and August; so, like its sister taxon
Known distribution. Confirmed to occur from the eastern Gulf of Alaska (La Chausee Spit) to the tip of the Alaska Peninsula (Cold Bay, Sanak Island) (
Comments. An earlier record of this species from Prince William Sound was published as
>
Pyropia taeniata S. C. Lindstrom sp. nov. (
This species was identified as Unknown #2 in Lindstrom (2008).
Description. Thalli lanceolate to linear, usually tapering to a point when young, with finely ruffled margins (male thalli occasionally planar), reaching at least 44 cm in length and 14 cm in width although usually shorter (20-40 cm) and much narrower (1.2-3.0 cm); often distinctly stipitate. Monostromatic, with a single chloroplast per cell. Adhering well to paper. Color usually reddish pink, reddish brown, or reddish purple. Male and female reproductive cells usually on separate plants; when on the same thallus, sectored by a vertical line (UBC A90660) or even monoecious, with marginal spermatangial streaks adjacent to zygotosporangial patches (UBC A90661, A90669, A91706, A91709, A91711, A91742, A91743, A91744, and A91752). Spermatangia in a narrow band along the margin, occurring according to the Hus formula 2 /a, 2-4/b, 8/c; spermatangial thalli 45-70 μm thick. Zygotosporangia in reddish distal ends and along distal margins, becoming hieroglyph-like or mottled as spores are released, arranged according to the Hus formula 2-4/a, 2-4/b, 4/c; zygotosporangial thalli 45-50 μm thick.
Holotype. UBC A90672, Sunshine Cove, Lynn Canal, Southeast Alaska, low intertidal bedrock, Apr 19, 2011, Leg. S. C. Lindstrom 14611. GenBank KT936189. The holotype consists of two thalli, one spermatangial, the other zygotosporangial.
Isotypes. There are no isotypes, but there is abundant topotype material in UBC and ALAJ, the herbarium of the University of Alaska Southeast, Juneau (see Comments below).
Etymology. The specific epithet refers to the fine, ribbon-like nature of the thallus.
Habitat. This species occurs on low intertidal rock, usually bedrock or boulders, except in the western Aleutian Islands, where it occurs in the mid to high intertidal and may also occur epiphytically.
Phenology. This is a spring species, extending into summer in colder regions. Most specimens were collected in April, May, and June, but July and August collections were also made in the Aleutian and Pribilof Islands.
Known distribution. Northern Southeast Alaska (near Juneau), Gulf of Alaska west through the Aleutian Islands to Kamchatka (Lindstrom 2008).
Comments. This Alaskan species has been identified as
This species is very closely related to the North Atlantic
>
Pyropia unabbottiae S. C. Lindstrom sp. nov. (
This species was identified as Unknown #1 in Lindstrom (2008).
Description. Thallus broadly lanceolate to nearly orbiculate, to at least 30 cm tall and 20 cm wide but usually smaller. Margin very strongly ruffled, especially near the base (the distal end is often quite flat), the ruffles extending nearly to the center of the thallus, especially mid thallus. Center of proximal part also planar, often greenish, whereas rest of blade is dusky pink or purplish. Older thalli often extremely perforated (presumably from littorine grazing and subsequent growth of the blade). Monostromatic, with one chloroplast per cell. Adhering well to paper. Vegetative and reproductive thalli c. 50-60 μm thick. Male and female structures intermixed on same thallus, occurring along a broad marginal area in the distal region of the thallus. Spermatangia in streaks or distinctly patterned patches amid solid red to pale zygotosporangial areas, the solid red areas reminiscent of
Holotype. UBC A90681, Akun Bay, Akun Island, low intertidal boulder, Jun 13, 2008, Leg. S. C. Lindstrom 13780. GenBank KT936200. The holotype consists of two thalli, both of which are fertile.
Isotypes. SCL 13781 in UBC (A90677). GenBank KT93 6203.
Etymology. The name is a combination of Unalaska, where many specimens were first collected and identified, and
Habitat. This species usually occurs on lower mid intertidal bedrock or boulders usually in areas subjected to some oceanic swell. It occasionally can be found higher or lower on the shore. It can also occur epiphytically on
Phenology. Like its sister species,
Known distribution. Vancouver Island (near Victoria and Malcolm Island), Kenai Peninsula (Chugach Bay), and the Aleutian Islands (Akun Island to Attu Island) (Lindstrom 2008) (Table 2,
Comments. When co-occurring with
Recent studies of the Bangiales on a global scale (Sutherland et al. 2011) as well as more localized studies in the northeast Pacific (Lindstrom 2008, Kucera and Saunders 2012, Lindstrom et al. 2015) allow us to update the distributions of the following species of Bangiales confirmed herein to occur in the Aleutian Islands:
1) Filamentous Bangiales. Two species of filamentous Bangiales have been confirmed from the Aleutian Islands. One, in ‘Bangia 2’ (
2) Foliose Bangiales.
3)
4)
5)
We were unable to confirm the occurrence of the following species in the Aleutian Islands:
There is a possibility that some of the Aleutian Bangiales are the same as some of the poorly known species from the northwestern Pacific Ocean. The northern Japanese species of
Although the Aleutian Islands offer a near-ideal habitat for species of Bangiales (cold, high disturbance, limited herbivory), the numbers of species we were able to confirm is surprisingly low (2 filamentous and 15 foliose species). We suspect that a number of species have yet to be uncovered in the flora. Collecting opportunities are largely limited to summer months (May to August), and this may in part be responsible for these low numbers. However, the possibility remains that these numbers reflect the true diversity of Bangiales in the Aleutian Islands. Only further intense collecting will determine which is the case.