Oak silkworms,
Cocoon material is composed of two kinds of proteins, a core protein (fibroin) and a gum-like protein (sericin). The content of sericin in the cocoon is different among the silkworm cocoons spun by silkworm kinds. To use
In this study, we examined the condition of
The cocoon was degummed with physical method using autoclave and chemical methoud using various concentration of sodium salt and then washed with distilled water. Degumming loss percentage was calculated according to the following equation.
Degumming loss percentage (%) = (Wi – Wf) / Wi × 100
Where, Wi is initial weight of dry cocoon; Wf final weight of dry cocoon.
Ultra violet spectrometer (Lambda 10, Perkin Elmer, USA) was used to measure the UV absorbance patterns of degummed
Fourier transform infrared (FT-IR) absorbance spectra were obtained using FT-IR spectrometer (Spectrum 100, Perkin Elmer, USA) in the spectral region of 1800 ~ 1000 cm-1 at a resolution of 2 cm-1 and 32 repeated scans were averaged for each spectrum.
>
Degumming condition of A. yamamai silkworm cocoon
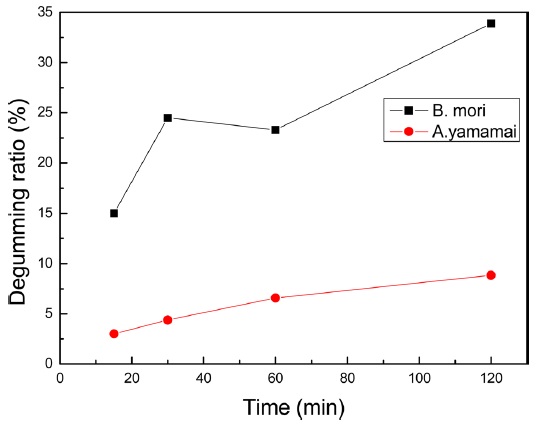
To impose unique silk fabrics characteristics, raw silk has been degummed during the textile processing. Soap degumming is one of a common method for domestic silk degumming. The main component of soap is sodium oleate. Fig. 1 showed degumming ratio of
Wild silkworm
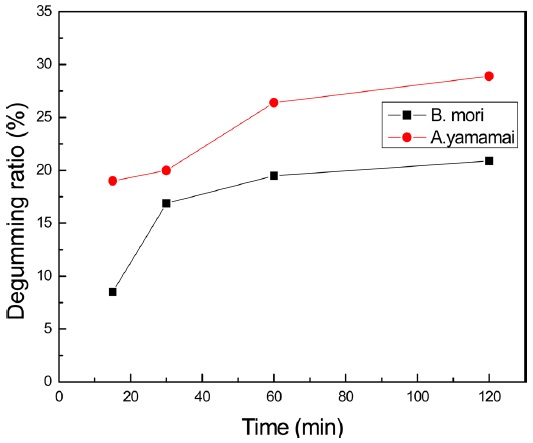
Fig. 2 showed degumming ratio of
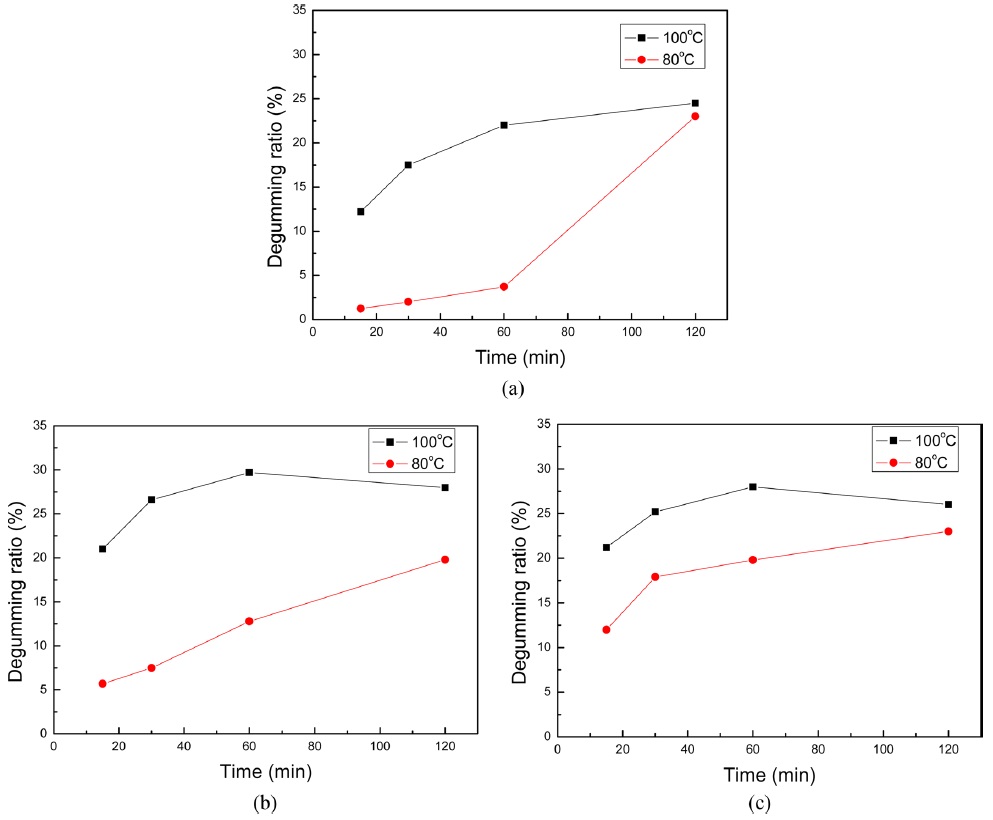
Fig. 3 showed degumming ratio of
Degumming ratio was reported various researchers using different degumming process. Shin
>
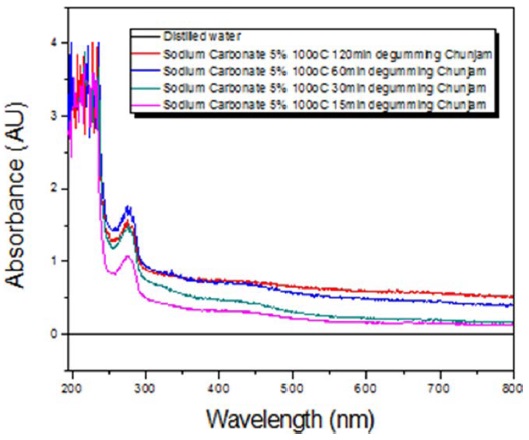
UV spectra of A. yamamai sericin
>
FTIR spectra of degummed A. yamamai cocoon
FT-IR is a powerful technique to examine the conformation of protein. In particular, the position and intensity of amide bands are sensitive to molecular conformation of silk protein. The spectrum of wild silkworm cocoon showed strong absorption band at 1650 cm-1 (amide I), 1530 cm-1 (amide II), attributed to the α-helix conformation (Kweon
The degumming condition for