The analysis of nuclear materials, environmental samples, and nuclear forensic samples is important for nuclear safeguards and security. The analysis of nuclear safeguard samples has become an important issue in the international community since the identification of undeclared nuclear activities in Iraq and North Korea in the early 1990s by the International Atomic Energy Agency (IAEA).1-2 The IAEA started to implement swipe sample collection and analysis as an additional routine process in facility inspections to verify declared activities.3-4 The collected samples have been analyzed at the IAEA Safeguards Analytical Laboratory (IAEA-SAL) as well as at the IAEA Network of Analytical Laboratories (IAEA-NWAL), designated by the IAEA to accurately estimate the actual nuclear activity of its member states. Currently, regarding environmental sample analysis, the IAEA-NWAL has 18 member laboratories including the Korea Atomic Energy Research Institute (KAERI). An environmental sample analysis can provide additional measures on the identification capability for nuclear activities. The analysis of environmental samples is conducted in an attempt to verify declared nuclear activities, as well as to detect undeclared nuclear activities. Therefore, swipe samples are typically collected from inspected nuclear facilities and are analyzed using various analytical methods. In an environmental sample, analysis of isotopic ratios, for U and Pu in swipe samples using mass spectrometry, represent our primary interest. The isotope ratios of 234U/238U, 235U/238U, and 236U/238U are of particular interest, along with 240Pu/239Pu.
Meanwhile, nuclear forensics has been developed in response to frequent incidents involving the illicit trafficking of nuclear materials around the world at the beginning of the 1990s. The frequent illicit trafficking of nuclear or radioactive materials is partly attributable by the loose control of nuclear materials during the breakdown of the former Soviet Union. Over the last 20 years, more than 2,000 incidents involving nuclear or radioactive materials have been reported to the ITDB (2015 IAEA Illicit Trafficking DataBase).5 Due to increasing illicit trafficking incidents involving radioactive materials, the Nuclear Forensics International Technical Working Group) was established to develop nuclear forensic techniques and provide a base for international collaboration. Over the years, ITWG has led on international technology developments and provided basic guidelines for nuclear forensic applications. Nuclear forensics typically deals with unknown samples that are seized during illicit trafficking. The main purpose of nuclear forensics is to identify the origin and intended use of illicitly trafficked nuclear materials.6-8 Therefore, sample analysis is the most important component of nuclear forensics. Through such analyses, important data, such as the isotope ratios of U and Pu, impurities contained in the target samples, and production dates, can be obtained and used to trace the origins of seized samples.
Although these analysis techniques use similar analytical instruments and methods, as mentioned above, the purpose of the analyses may be very different. Mass spectrometry has been considered as the most effective analytical tool in such sample analyses, especially with repsect to isotopic analysis, age dating, and impurity analysis. Because mass spectrometry can provide important information on isotopic composition, elements, age dating, impurities, etc., its use in this field is increasing. Recent publications also highlight the growing interest in mass spectrometry in nuclear industries.9-11 In this report, important parameters in the analysis of nuclear safeguard samples, as well as in nuclear forensics, are discussed. In addition, application of the most common mass spectrometry methods, such as thermal ionization mass spectrometry (TIMS), inductively coupled plasma-mass spectrometry (ICP-MS), and secondary ion mass spectrometry (SIMS) for samples analysis are discussed in detail. Finally, similarities and differences, as well as advantages, are compared between the two analytical methods.
Characteristics and role of mass spectrometry in environmental sample analysis
In a normal facility inspection by the IAEA, two types of samples can be collected: nuclear material samples and swipe samples. Nuclear material samples are typically process samples that can be produced during physical or chemical processes involving nuclear activities such as reprocessing, enrichment, and fabrication, or during the process of analyzing nuclear materials or fuels. Since they are process samples, the radioactivity levels - and content of nuclear or radioactive materials - in the target samples are high. Meanwhile, swipe samples are collected during the inspection process using cotton cloths; such samples typically contain a much smaller amount of nuclear material, and sometimes only a trace amount. Figure 1 shows a picture of a swipe sample. Therefore, the analysis methods differ between these two sample types. For the analysis of nuclear materials, mass spectrometry and radiometric detection - such as alpha spectroscopy and gamma spectroscopy - are the most common methods.12-13 Radiometric methods are typically preferred for nuclear material analysis owing to the simple non-destructive measuring process. However, it takes longer to measure samples with a low activity while still obtaining sufficient analytical reliability. Mass spectrometry can also be used for the analysis of nuclear materials, to precisely determine the isotopic composition. In a swipe sample analysis, radiometric detection can be used only for screening nuclear materials owing to its poor measurement sensitivity. In a detailed analysis of environmental samples, mass spectrometry is the most important method for determining their contents, and the isotope ratios for U and Pu. The presence of a 236U isotope in samples is suggestive of reprocessing of the irradiated fuels, since there is no 236U isotope in natural U samples. In addition, the isotope ratio provides information regarding the type of reactors used for irradiation. The isotope ratio of Pu is also indicative of the reprocessing activity of irradiated fuels.
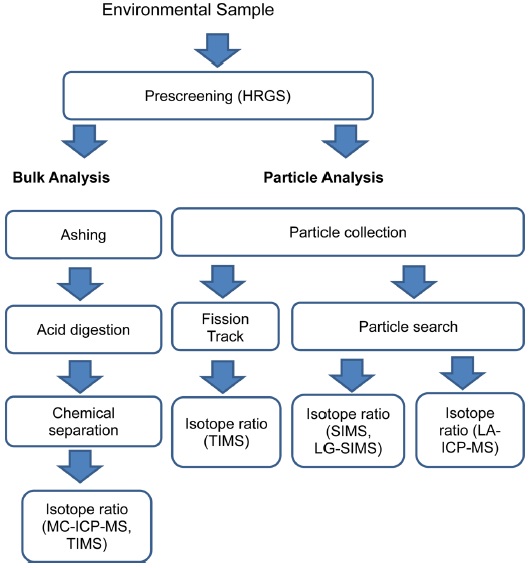
In the analysis of swipe samples, it is important to consider an efficient sample analysis process since such samples have a very limited availability and a small amount of nuclear material. Because the swipes contain a very small amount of nuclear material, analytical methods such as chemical separation and isotope measurement procedures must be adjusted to deal with an ultra-trace amount of sample. In this swipe sample analysis, two methods - namely, bulk analysis and particle analysis - have been developed. Various analytical techniques and mass spectrometry methods have been applied in this field, and novel techniques have been developed. Figure 2 presents a general analytical procedure for nuclear safeguard samples.
A bulk analysis can provide information on the average isotopic composition and content of nuclear materials contained in swipe samples.14 In this method, sample swipes are ashed using a high-temperature oven or furnace. The prepared ashes are then treated with appropriate acids such as high purity HNO3 or HCl to completely dissolve the sample ashes. The dissolved samples are then chemically separated using various chemical separation methods such as anion exchange resin, extraction chromatography, or precipitation.15-18 Kim et al. adopted TEVA-Spec and Sr-Spec for U separation and Pu concentration from several certified or standard reference materials.15 Takahashi et al. reported on the chemical pretreatment methods for swipe samples using an anion exchange resin.16 Lee et al. adopted UTEVA resin for the separation of U and Pu from simulated samples.17 Amstrong et al. also reported the use of anion exchange resin for the separation of U and Pu in swipe samples.18 Recent trends indicate that each laboratory develops its own procedure by combining an anion exchange resin, UTEVA, and TEVA columns. Once chemical separation of the sample solution is complete, it is possible to measure the isotopic ratio and contents.
The measurement of the isotopic abundances and contents of each separated portion can be determined using mass spectrometry. Isotope dilution mass spectrometry (IDMS) is generally adopted for accurate determination of the sample content. In the IDMS approach, 233U and 242Pu are mostly adopted as spikes for U and Pu measurement, respectively. In a bulk analysis, TIMS has been most commonly used due to the low degree of mass spectral interference and mass bias, as well as the high precision and abundance sensitivity with a multi-collector configuration.19-23 The majority of TIMS systems have several Faraday cups along with one or a few secondary electron multiplier (SEM) detectors. Therefore, a simultaneous detection of isotopes can be performed. In 1992, McCormick reported the use of TIMS in the isotopic analysis of U and Pu19 and was able to obtain an accuracy of 0.2%. Aggarwal’s recent review discusses the use of TIMS in nuclear material analysis.11 Oliveira et al. used TIMS in measuring the isotope ratios of 235U/238U for IRMM (Institute for Reference Material and Management) isotope reference materials. They presented a relative expanded uncertainty in the range of 0.11-0.14%.20 Park et al. adopted the TIMS technique for an ultra-trace analysis of U for the U030 sample, and Pu for a REIMEP-16A round-robin sample, respectively.21 A total of 5 pg of U030 and 1 pg of REIMEP-16A were used for isotope analysis. The accuracy of this type of measurement was less than 2% for simultaneous ion detection using several multi-ion counters (MICs). In a recent study by Saito-Kokubu et al., TIMS was applied for isotope analysis of U and Pu in U-Pu mixture samples by adopting a continuous heating method.22 Using this approach, the relative standard deviation of the isotope ratio in a sample of U/Pu ratio ≈ 1 was less than 2% for 240Pu/239Pu and 3~4% for 235U/238U. Park et al. also applied the TIMS system to validate bulk analysis of the environmental swipe samples.23 Recently, Lee et al. reported a method to independently detect U isotopes and Pu isotopes from a mixed particle (U+Pu) sample by varying the thermal ionization conditions using a continuous heating method.24 This technique provides a solution to the analysis of isobaric elements or molecules contained in an environmental sample analysis. The minimum detectable amount of U and Pu samples for determining isotopic compositions are a few hundred pg and a few pg, respectively.
Another mass spectrometry approach used for isotopic analysis is ICP-MS, which has become one of the most common mass spectrometry approaches for isotope ratio measurement of nuclear materials.25-29 ICP-MS utilizes high-temperature plasma for atomizing and ionizing samples. Therefore, the ionization efficiency and detection sensitivity are high compared to TIMS. The detection of ions in ICP-MS is normally performed using Faraday cups or high performance ion counters, as was the case for TIMS. A recently developed multi-collector (MC) system provides high detection sensitivity along with an easy sample introduction. Nevertheless, the abundance sensitivity remains poor compared to the TIMS system owing to the broad mass spectral width caused by the wider kinetic energy distribution of ions. Interference from molecular complexes and difficulties in cleaning the sample introduction area are other disadvantages of this approach. Magara et al. reported an analytical technique for environmental samples using a double focusing sector type ICP-MS and New Brunswick Laboratory Certified Reference Material (NBL CRM U350) as the sample.25 Farmer et al. adopted ICP-MS for an environmental sample analysis of Pu and U.26 Godoy et al. reported the application of inductively coupled plasma-quadrupole mass spectrometry (ICP-QMS) for the sensitive detection of Pu isotopes in sediment samples.27 They adopted a TEVA column for chemical separation. Szeles et al. reported tan environmental sample analysis using high resolution inductively coupled plasma-sector field mass spectrometry (ICP-SFMS)28 and used extraction chromatography with TRU resin for chemical separation.
The recently developed MC ICP-MS shows improved performance of the ICP-MS technique, making it a popular instrument for various analytical applications. The MC system allows simultaneous detection of isotopes of U or Pu and a higher detection sensitivity, up to the ppt level. When MC-ICP-MS is adopted for bulk analysis of environmental samples, a de-solvation system, such as the Aridus (Teledyne; CETAC Technology) or Apex (CPI international) system, is also interfaced with a mass spectrometer as a sample introduction technique. Lim et al. reported the use of MC-ICP-MS for validation of a bulk analysis of simulated swipe samples. They used UTEVA columns for chemical separation and Aridus II was employed as a desolvation system.29 Pestana et al. reported a new swipe sample analysis method based on ultrasound-assisted acid leaching using ICP-MS. In this approach, rapid sample preparation was achieved with an uncertainty of 2.5 to 3.4% for the 235U/238U ratio by leaching only the sample.30 Mitroshkov et al. explored the formation rate of a polyatomic species in a Pu analysis using MC-ICP-MS.31 Their system was equipped with the Aridus II desolvation system. The formation rate was estimated to be 1.1 × 10-8, 8.9 × 10-8, and 1.7 × 10-9 for 199Hg40Ar, 191Ir16O3, and 207Pb16O2, respectively. The majority of environmental sample laboratories are equipped with an MC-ICP-MS system, and they tend to use this system as a primary analytical instrument for bulk analysis. This is due to the high detection sensitivity and convenient sample introduction method of MC-ICP-MS. This easy sample introduction system provides a high throughput of sample analysis in a given time. Because an environmental sample analysis deals with ultra-trace amounts of nuclear materials, the low background of U or Pu is essential to achieve a good detection limit. In general, clean facilities with a low background uranium level are used in an effort to minimize sample contamination. In a normal laboratory environment, some U and Pu are present as a background, which can limit the measurement accuracy.32-33
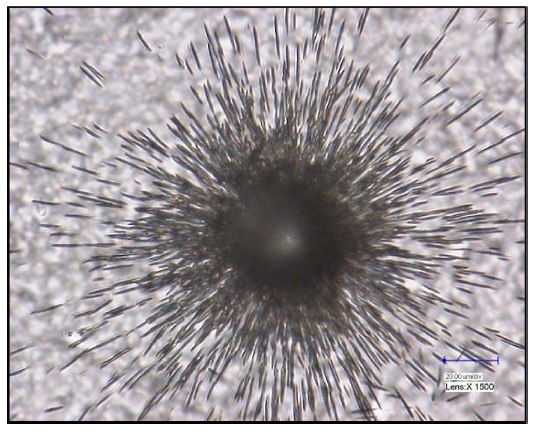
Particle analysis techniques are used to determine the isotope ratio of each nuclear particle contained in a swipe. Because this approach analyzes each individual particle, it requires an extended analysis time and a more sophisticated analytical technique.34-35 To perform particle analysis, particles from the cotton swipe have to be collected. Among the available techniques, vacuum impactors and vacuum filtration are the most commonly used.36-37 In the vacuum impaction method, the collection of particles can be performed on a graphite planchet for subsequent SIMS analysis, while the vacuum filtration system is equipped with a membrane filter for use in fission track thermal ionization mass spectrometry (FT-TIMS). In particle analysis, the pre-screening process is required to pinpoint the existence and location of target nuclear materials. For this purpose, the fission track (FT) method has been used for FT-TIMS analysis. The FT is generated from the neutron-activated nuclear materials, and detectors such as Lexan and Markrofol can be used to record the FT.38-42 An example of fission track generated by a U particles is shown in Figure 3. TIMS has been commonly used for particle analysis in combination with the FT-TIMS screening technique. The FT-TIMS technique is considered one of the most accurate and reliable particle analysis methods since it can identify exact nuclear particles using the FT technique combined with the superb performance of TIMS for isotope ratio measurement.43-46 The identified nuclear particles are selected, cut using a micromanipulator, and loaded onto the TIMS filament for mass analysis. In addition to FT-TIMS, the SEM-TIMS method has been developed for particle analysis, by using TIMS without a research reactor. In the SEM-TIMS technique, particle identification is performed using energy-dispersive X-ray spectroscopy equipped in an SEM system (SEM-EDX).47 Kraiem et al. and Park et al. recently applied the SEM-TIMS technique for single particle analysis for nuclear safeguard purposes.48-50 However, the use of SEM-TIMS is not very popular compared to the FT-TIMS or SIMS techniques, owing to the difficulty in transferring target particles to the TIMS filament or carbon planchet for SIMS analysis.
SIMS is commonly used for particle analysis due to the easy analysis of particle samples. However, a small geometry SIMS system, which is a normal SIMS with a small mass analysis geometry, has disadvantages such as a low mass resolution, difficulty measuring the Pu isotope ratio, and poor measurement sensitivity compared to FT-TIMS (although it shows a rapid analysis time compared to the FT-TIMS method). Because the SIMS method has been the only available particle analysis method other than FT-TIMS, many technical advancements have been made in applying this technology for safeguard sample analysis. Tamborini et al. described the application of SIMS in a single U particle analysis using NBS U005, U010, and U030 particles.51 They compared the measured result with the FT-TIMS results; the measured result was comparable with the FT-TIMS data and a detection limit of ng/g-pg/g was reported. Esaka et al. adopted the SEM-SIMS technique with particle isolation to measure the isotope ratio of the reference particles.52 Although they were able to isolate U particles from Pb particles, the molecular ion interference prevented an accurate isotope ratio measurement from being obtained. Esaka et al. applied the SIMS technique to investigate the dependence of precise U isotope ratio measurements on particle diameter.53 One of the required techniques for SIMS analysis of a particle is a method to locate the position of the nuclear particles with raster scanning. This has been accomplished using SEM-EDX FT and other particle search techniques. Recently, Cameca Inc. developed an automated particle searching system, called the Automated Particle Measurement (APM) software. This software provides efficient single particle analysis capability to small geometry SIMS systems, such as the CAMECA (6f and 7f models).54 Another particle analysis technique using SIMS is FT-SIMS, which was reported by Esaka et al.55 This technique allows for efficient particle identification of highly enriched uranium in environmental samples. Esaka et al. also applied APM screening for the analysis of U particles using TIMS.56 Shinonaga et al. adopted an SEM system, along with ICP-MS and TIMS, for isotope measurements of U and Pu particles. The manipulator was installed in the SEM for selection of target particles using manipulators. The selected particles were dissolved in acid for mass spectrometric measurements.57
New development in particle analysis techniques
New technical developments have been achieved in particle analysis using SIMS by adopting an MC system and large geometry (LG) for high mass resolution. This new SIMS is typically referred to as LG-SIMS.58-59 Combining the high mass resolution of LG-SIMS and the particle search capability of APM allows for a rapid particle analysis system using the SIMS technique with limited isotopic interference. Although the performance of LG-SIMS and normal SIMS have shown significant improvements, there are still weaknesses in the SIMS techniques compared to FT-TIMS, such as difficulties in analyzing Pu isotopes. This is because a contribution of hydride to the mass spectra is inevitable using the SIMS technique. Hydride generation leads to overlap of the mass spectrum with molecular hydrides and prevents clear identification of the isotopes of nuclear materials from mixed materials. In addition, the high price of the instrument may limit its availability. Nevertheless, use of the LG-SIMS system for particle analysis is becoming more common, despite the high price of the instrument. This phenomenon indicates that the need for a rapid particle analysis system is increasing.
ICP-MS has been used for both bulk analysis and particle analysis. Two techniques are available: ICP-MS analysis followed by the dissolution of particles in a solution, and laser ablation ICP-MS. Zhang et al. studied the use of ICP-MS for isotope ratio determination of U miocroparticles.60 They isolated each U particle and dissolved them into solution before measuring the isotopic ratio using ICP-MS. Esaka et al. also introduced a particle analysis technique for U by adopting ICP-MS with nebulization along with FT or APM particle-searching techniques.61 This approach is an alternative to particle analysis with the ICP-MS system. Laser ablation ICP-MS (LA-ICP-MS) has only recently been adapted for particle analysis and solid sample analysis for nuclear safeguard applications.62-63 Varga studied the LA-ICP-MS technique for analysis of a single U particle.62 A relative standard deviation of 0.95 to 5.1% for major isotopes was reported. Kappel et al. performed isotope ratio measurements of a single particle using LA-MC-ICP-MS.63 The adopted particles were U oxide particles (9073-01-B, NBL CRM U010, and NUSIMEP-7 test sample (IRMM-184)). Pointurier et al. applied LA-ICP-MS for isotope analysis of single microparticles64 and analyzed NUSIMEP-6 and NUSIMEP-8 round-robin particle samples and IAEA swipe samples. Based on their results, there is a potential advantage of LA-ICP-MS combined with FT, but further studies are required before it can be applied for particle analysis. In the laser ablation process, the nanosecond (ns) laser system is typically adopted for ablation of the sample and introduction of the ablated sample to the ICP plasma, while the femtosecond (fs) laser system represents a new trend in LA-ICP-MS.65 The use of an fs laser in the ablation process has enhanced the precision and accuracy of the isotopic analysis. Since this technique has a much lower matrix effect compared to the SIMS technique, and a higher sample analysis throughput, rapid technical development is expected in the near future. Another concern pertaining to particle analysis techniques is the size of the sample particles. If the particle is too small, the detection sensitivity limits measurement of the isotope ratio of the sample particle, due to the limitations of mass spectrometry. In the FT-TIMS analysis, the smaller size of the particle prevents the identification of nuclear materials using a fission track. In an SIMS particle analysis, the analysis of particles smaller than 1 μm is also difficult. Nevertheless, particle analysis techniques for environmental samples are continuously advancing toward achieving a higher detection sensitivity, high mass resolution, and low hydride contributions.
Characteristics and role of mass spectrometry in nuclear forensics
>
Characteristics of nuclear forensics
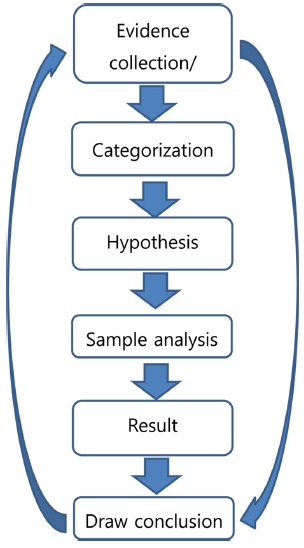
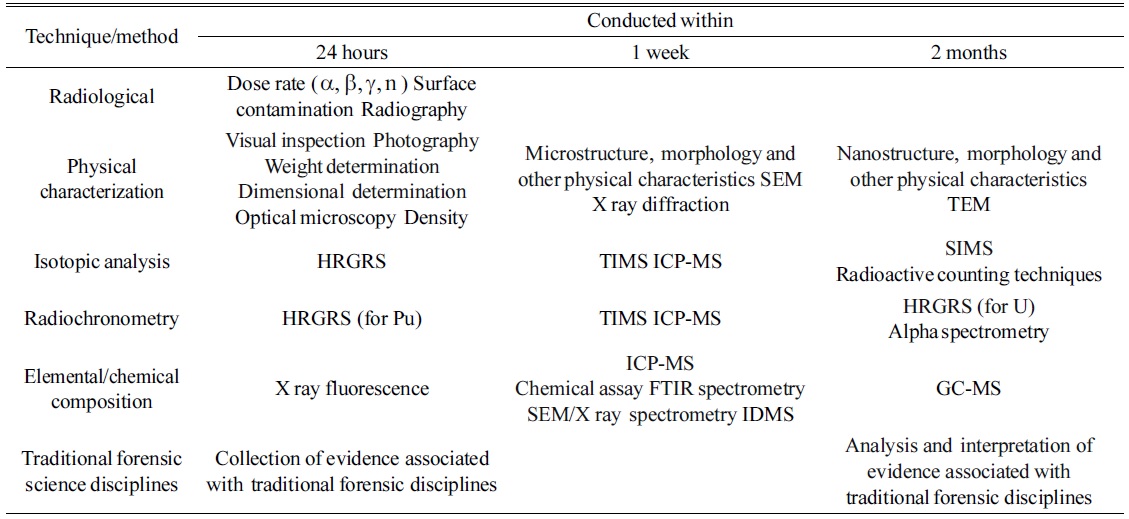
Samples collected in a nuclear forensics investigation can have many different shapes, activities, and compositions, which differ from those of environmental samples. In this regard, analytical methods can differ depending on the characteristics of the sample. Therefore, a nuclear forensics analysis has a different procedure from a safeguard sample analysis. Figure 4 shows the general procedure for nuclear forensics. Once the sample is collected, the hypothesis can be derived based on the gathered information; then, the sample analysis can be performed. After analysis, the results are compared to the hypothesis, and this step can be repeated until the analysis data can be correctly interpreted. The application of mass spectrometry is important for the sample analysis process. As mentioned above, nuclear forensics attempts to determine the origin of the seized samples. Therefore, the isotope ratio of nuclear materials, stable isotopes, decay products such as Pb, Sr, and Nd, and rare-earth element distribution patterns are important parameters. In addition, most forensic analyses dealing with nuclear materials are associated with a criminal act such as theft of a material, illegal transportation, or illegal use. Therefore, rapid analysis of seized samples is required. Such analysis starts with identification of the nuclear activity of the seized samples. Three-step analyses - i.e., a 24-hour, 1-week, and 2-month analysis - are commonly applied, as suggested by the IAEA and ITWG.66 In a 24-hour analysis, an estimation of the radioactivity and physical characteristics, and a rough estimation of the isotopic composition, are important. The image, odor, color, density, and nuclear activity of the samples should be measured in a 24-hour analysis process. Isotopic measurements can be also performed during the 24-hour measurement step. Once the sample is identified as nuclear or radioactive material, the nuclear forensics system can be applied to estimate the origin of the seized material. In a 1-week analysis, a detailed isotopic analysis, structural analysis of the sample, and impurity analyses are important parameters. In a 2-month analysis, a detailed and thorough isotopic analysis, including chemical separation, age dating, and stable isotope analysis, can be performed. Table 1 lists the analytical methods adopted in a nuclear forensics analysis. Kristo and Turney presented a three-step analysis process for ITWG round-robin samples.67
[Table 1.] Laboratory methods and techniques with typical timescales for completion of analysis66
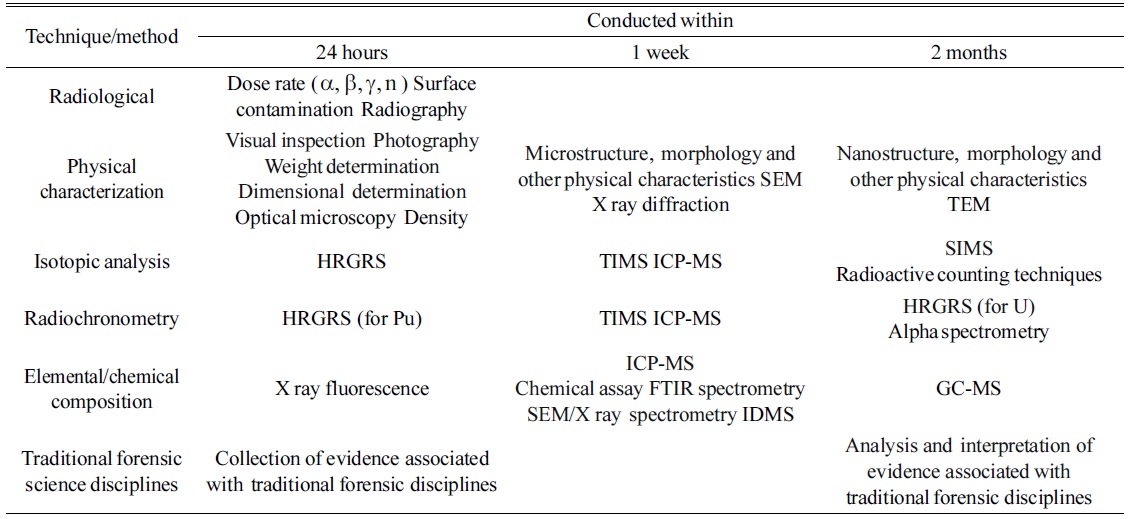
Laboratory methods and techniques with typical timescales for completion of analysis66
Age dating is an important parameter in nuclear forensics, as well as in nuclear safeguards, because the age dating process can determine the date of separation of U or Pu from the original material. The age dating technique has been developed based on the decay of nuclides. When radioactive nuclides decay to fission products, both mother nuclides and daughter nuclides are present in the sample. If the isotope ratios and contents of these nuclides can be accurately measured, the date of the separation can be easily estimated using a decay equation. For isotope analysis or age dating of nuclear materials, chemical separation of the nuclear materials is important for obtaining an accurate analytical result. The chemical pretreatment and separation processes differ among nuclear forensics samples, because the type of sample - and the content of the nuclear material - vary significantly, from trace samples to scrap metals and spent fuel. In nuclear forensic samples, impurities, rare-earth elements, and age dating are also important parameters requiring measurement. Although there are many examples of chemical processes used for the analysis of nuclear materials, only some examples are discussed. The separation of U and Pu isotopes from coral soils, contaminated by nuclear weapon testing, has been analyzed using both a UTEVA and TEVA column.68 Chemical treatment and separation for age determination of U-Pu mixed particles with a variable U/Pu ratio (1~70) was reported by Miyamoto et al.. They used a sequential anionic exchange resin for chemical separation.69
Mass spectrometric application in nuclear forensic analysis
In nuclear forensics, mass spectrometry in an environmental sample analysis is an essential tool that can be applied for isotopic analysis of nuclear materials, impurities, and rare earth elements. TIMS, ICP-MS, or SIMS are popular choices due to their availability and convenience. TIMS has been used for the isotope analysis of nuclear materials and nuclear forensics samples.70-71 Wallenius et al. reported detailed results of investigations of pellet samples and uranium powder, including isotope ratio, age determination, and impurities, using TIMS and ICP-MS.72 The detection limit of TIMS system nuclear forensics samples has been reported to be lower than 100 ag for Pu analysis when the cavity ion source is adopted for sample vapor generation.73 The cavity ion source provides extended sample evaporation times to improve the detection limit. A recent study by Lee et al. indicated that 6 ag of Pu can be analyzed.74 They used a continuous heating method with a CRM 947 sample. TIMS has also been used for origin determination of Pu samples from spent fuel.75 This previous report contains data on age determination and the isotopic correlations between samples and types of reactors. TIMS has also been adopted for age dating of Pu samples.76 Recently, TIMS has been used to measure the isotopic composition and concentration of U and B.(77) The adopted spikes for IDMS were U-233 and B-10. TIMS has been used for the detection of minor U isotopes by Quemet et al. and they obtained less than 5% uncertainty for a 234U/238U isotope ratio with 50 ng samples using a peak jumping method with an electron multiplier.78 They concluded that the use of a multi-ion counter (MIC) or SEM can improve the detection sensitivity, and decrease the analyzable quantity, from 1,270 ng for a Faraday cup-only case to 50 ng for cases of a Faraday cup with SEM, or a Faraday cup with MIC. Although ICP-MS is more commonly used in isotope analysis, TIMS remains one of the best-performing mass spectrometers in a nuclear material analysis of environmental samples and nuclear forensics analysis.
ICP-MS has been used for the analysis of various nuclear forensic samples, such as U pallets and uranium ore concentrates (UOCs). Mironov et al. reported burn-up of irradiated reactor fuel in an analysis of the isotope ratio in soil samples using ICP-MS.79 Isotope ratios of Pb, Sr, and Nd, as well as the rare earth element distribution in the sample, provide information on the sample origin. Varga et al. studied the Pb and Sr isotope ratios for origin assessment of UOCs.80 Varga and Suranyi reported on the detection of previous neutron irradiation and reprocessing of nuclear materials by analyzing the U isotopes of materials with ICP-MS.81 Krajko et al. used Nd isotope measurement data, obtained using ICP-MS, for the origin assessment of UOCs.82 Keegan et al. reported on the nuclear forensic analysis of UOCs seized in Australia using ICP-MS.83 Varga et al. reported on the rare-earth element patterns in U-bearing material using ICP-MS.84 The isotope ratios of 234U and 236U relative to 238U have been accurately measured in those reports. LA-ICP-MS has been used for isotope analysis and rapid categorization of uranium oxide materials.85 Marin et al. used LA-SF-ICP-MS for nuclear forensic analysis of an ITWG round-robin-3 sample which were used for the third round-robin organized by ITWG.86 ICP-MS has also been commonly used for age dating of nuclear materials, and age dating of corals has been performed using LA-MC-ICP-MS.87 The age dating of Pu reference solutions (IRMM083 and IAEA367) was reported by Nygren et al. who used 241Pu/241Am and 240Pu/236Pu chronometry and ICP-SFMS.88 Round-robin results of 230Th-234U age dating were reported by Gaffney et al.89 Varga et al. performed Pu age dating by adopting ICP-MS.90 The analysis of impurities in U-containing samples has also been the focus of nuclear forensic analysis. Han et al. reported on the variation of sulfur isotopes in various UOCs, and showed that the isotope ratio of S can be used to determine the sample origin.91 Subsequent to that report, Krajko et al. reported on sulfur isotope variation due to different processes during UOC production.92 Since the isotopes of several elements can indicate the sample origin, further studies are required on the impurity of various U-bearing samples such as UOCs, U mines products, and U pallets.
SIMS has also been used for the analysis of nuclear forensic samples. Betti et al. reported using SIMS for characterization of Pu and highly enriched U particles with a diameter of 10 mm.93 The isotopic compositions of particles were measured to within 0.5%. They claimed that their SIMS measurement was compatible with data obtained using TIMS. This group also utilized the SIMS technique for the characterization of radioactive particles, and were able to identify hot particles that originated from the Chernobyl accident in an IAEA 375 soil sample.94 Tamborini published a report on the SIMS analysis of U and actinides in microparticles of different origins.95 Tamborini et al. also reported on the isotopic ratio using SIMS in uranium oxide microparticles. The isotope ratio of 18O/16O was measured with an uncertainty of 0.3 to 0.9%.96 The ITU group used the SIMS (Cameca 6f) system for these studies. Faure et al. reported on the detection of trace fluorine in U microparticles using SIMS.97 Fahey et al. reported on the origin of a U sample by measuring the Pb isotopic abundances.98 Hedberg et al. reported multiple ion counting measurements using LG-SIMS for nuclear forensics and safeguard analysis.99
Other types of mass spectrometry
For an environmental sample analysis, other spectrometry techniques are rarely adopted due to the particular requirements of swipe analysis, such as high precision and accuracy. However, methods such as resonance ionization mass spectrometry (RIMS) and isotope ratio spectrometry (IRMs) can be adopted for specific analytical purposes. RIMS can utilize the resonance enhancement of ion signals by applying specific wavelength laser beams to the samples before the sample ions are detected and analyzed using the time-of-flight mass spectrometry (TOF-MS) system. Therefore, RIMS can be applied for ultrasensitive and selective detection of elements or isotopes.100-101 Nuclear forensics samples can have more complicated characteristics, which renders the performance of a precise analysis more difficult. Laser ionization followed by ion beam sputtering, which is called sputtered-neutral mass spectrometry or laser post-ionization mass spectrometry, can act as auxiliary methods for the independent detection of U or Pu from complicated samples. IRMS has been applied for stable isotope analysis (e.g., for 14C, 15N, 18O, and 34S).102-103 Information on stable isotopes is an important aspect in determining the origin of a nuclear material, since geologically different locations typically show different isotope ratios for these stable isotopes.
Growing concern regarding nuclear safeguards and nuclear security has accelerated the development of nuclear sample analysis techniques. Mass spectrometry has been the analytical instrument of choice for nuclear material analysis, environmental sample analysis, and nuclear forensics. For these purposes, TIMS, MC-ICP-MS, and SIMS have played an important role. Laser ablation ICP-MS may be an alternative for particle analysis of an environmental sample and solid sample analysis in nuclear forensics. LG-SIMS is becoming an important instrument for particle analysis with MC capability, while RIMS can be an additional measure in a swipe sample analysis, especially for isobaric interference measurements. However, IRMS can provide additional information on the origin of nuclear forensic samples.