Theory of mind (henceforth, ToM) is the ability to attribute mental states (intentions, desires, emotions, thoughts and focus of attention) to other people, and to explain and predict their behavior on the basis of this attribution (Premack & Woodruff, 1978; Baron-Cohen, Ring, Moriarty, 1994; Happè, Brownell & Winner, 1999). This ability is involved in a wide range of everyday activities, such as understanding social situations, comprehending communicative exchanges, emotions and social conventions. As a consequence, impairments are strongly disabling (Frith & Frith 1999), as shown in people with autism (Baron-Cohen, 1991; 1995; Frith & Happé, 1994; Happé, 1993) and schizophrenia (Frith & Corcoran, 1996; Corcoran, Mercer & Frith, 1995; Pickup & Frith, 2001). Clearly, ToM is not the only way in which we can understand and predict others’ behaviour. For instance, motor simulation processes have been shown to play a role in understanding some basic aspects of others’ actions, intentions and emotions (see Keysers and Gazzola, 2006 for a review). However, as soon as the complexity of the social situation and of the observed behaviour increases, ToM comes to plays a major role in understanding others’ mental states.
Several experimental findings have suggested that ToM is domainspecific, independent of general intelligence, with specific information processing demands (e.g., Adolphs, 2001), and also that it may rest on an innately predisposed, dedicated cognitive mechanism (e.g., Fodor, 1992; Frith & Frith, 1999; Leslie & Roth, 1993; Happé et al., 1999; Happé, Malhi & Checkley, 2001). We know from the literature on developmental disorders that in some pathological conditions ToM seems to be dissociated from other cognitive abilities. For instance, in autism ToM is heavily impaired, while the other cognitive abilities are usually preserved (Baron-Cohen, Tager-Flusberg & Cohen, 1993); on the contrary, Williams syndrome usually implies an intact ToM despite impaired theorizing ability in other domains (Karmiloff-Smith, Klima, Bellugi, Grant & Baron-Cohen, 1995). Further evidence has been provided by neuropsychological and neuroimaging studies. Several findings have shown specific, acquired ToM impairments in patients who have suffered a brain damage, such as a right hemisphere stroke (Happé et al., 1999), frontal lobe surgery (Happe et al., 2001), frontotemporal dementia (Lough, Gregory & Hodges, 2001) and in schizophrenic patients (Brunet, Sarfati, Hardy-Bailé, 2003; Sarfati, Hardy-Bailé, Besche & Widlöcher, 1997). In contrast, other researchers have claimed that ToM is a domain-general cognitive function, strictly linked to executive functioning (e.g., Carruthers, 1996; Frye, Zelazo, Palfai, 1995; Frye, Zelazo, Brooks, Samuels, 1996; Hughes, 1998; Perner, 1998; Perner & Lang, 2000).
A series of other considerations raised in developmental psychology suggests that ToM ability may not be a unitary cognitive ability, but might be made up of distinct functional components, each of which could rely on specific neurological networks (for a discussion, see Bara, Colle & Bosco, 2005). To cite the most important findings, young children develop the ability to understand desires/intentions, emotions, true and false beliefs in a fairly predictable order: until two years of age, infants explain other people’s actions by referring only to their volitional states, i.e., desires and intentions (this has been called “Desire Psychology”, Wellman 1991), that are, at that stage, mental states indistinguishable from each other (Astington, 1991; 2001; Bartsch & Wellman, 1995). Then, between the second and third year, children show a dramatic increase in the understanding of emotions as mental states, as they start to talk consistently about emotions as causes and consequences of other people’s behavior (Dunn, Brown & Beardsall, 1991; Oatley & Jenkins, 1996; Frye & Moore, 1991) 1 . They also start to reason in terms of true beliefs. Thus, starting from the age of three children explain other people’s actions by referring to volitional states, emotions and true beliefs: this stage has been called “Desire-Belief Psychology”, (Wellman, 1991). Finally, it is only starting from the age of three to four that children successfully manage false beliefs (for reviews see Flavell, 1999 and Wellman, Cross & Watson, 2001), that involve keeping in mind the contrasting representations of a real state of affairs and the false beliefs of the other individual. It has been argued that the stages of development children usually undergo (i.e. Desire Psychology; Desire-Belief Psychology; understanding of false belief), may result from the appearance of distinct mechanisms, rather than from the gradual enrichment of a single mechanism (Saxe, Carey, Kanwisher, 2004).
Although the issue is still controversial, the multicomponential nature of ToM seems to be supported by the results of several functional brain imaging studies. To make inferences about other people’s beliefs (or cognitive perspective-taking) and other people’s emotions (or emotional perspective-taking) activates at least partially different brain areas, such as the frontopolar cortex in the belief condition, and the orbitofrontal cortex and the ventromedial prefrontal cortex in the emotion condition (e.g., Hynes, Baird & Grafton, 2006; Shamay-Tsoory, Tibi-Elhanany & AharonPeretz, 2006). A recent model proposed by Shamay-Tsoory and colleagues (2010), derived from a series of lesional studies, distinguishes cognitive (e.g. the ability to make inferences about others’ beliefs and motivation) from affective (e.g., the ability to make inferences about others’ emotional states) subprocesses of ToM. Such model has been further supported by neuropsychological studies (e.g., Reniers, Corcoran et al., 2012). It has also been shown that the ability to attribute emotions to others relies at least partially on different brain areas with respect to the ability to attribute intentions. Indeed, although some brain regions are activated in both tasks, a set of brain areas are involved specifically in attributing intentions, namely the lateral orbitofrontal cortex, middle frontal gyrus, cuneus and superior temporal gyrus (Völlm, Taylor, Richardson et al., 2006; see also Chiavarino, Apperly & Humphreys, 2010, for similar findings about the ability to attribute intentions and desires).
To sum up, developmental psychology and neuroimaging studies have provided converging evidence for the assumption that ToM ability may have distinct functional and neurological subcomponents, dependent on the content of the ToM, such as emotions, intentions and beliefs.
1However, the precursors of the ability to understand intentions, desires and emotions may be found much earlier; infants are able to recognize the intentions behind simple actions (such as grasping an object) starting from the first months of life (Woodward, 1998, 1999,2003,2005), and are able to differentiate facial expressions of different emotions starting from six months (Caron, Carom & MacLean, 1988).
Theory of Mind Following Traumatic Brain Injury
A traumatic brain injury (henceforth, TBI) often produces lesions concentrated in the frontal and temporal lobes of the brain (e.g., Adams, Doyle, Graham et al., 1985; Levin, Mattis, Ruff et al., 1987), the areas responsible for ToM ability. The violent acceleration which the cranium undergoes in head trauma results in two types of cerebral lesions:ecchymoses, caused by the brute force of the impact, and axonal damage and tissular sufferance, caused by the overstretching of the neurons, the blood vessels, and their supporting structures. Neither type of damage is confined to the area of first impact, but typically diffused throughout the brain, although with preferential localizations that depend on the anatomy of the various structures involved. However, a TBI usually results in an impairment of several cognitive subsystems. Several recent studies have pointed out the presence of a deficit in ToM ability in TBI patients (e.g., Bach, Happé, Fleminger & David, 2006; Bibby e McDonald, 2005; Channon & Crawford, 2000; Dennis & Barnes, 2001; Dimitrov, Grafman & Hollnagel, 1996; Havet-Thomassin, Allain, Etcharry-Bouyx & Le Gall, 2006; Henry, Phillips, Crawford et al., 2006; McDonald e Flanagan, 2004; Milders, Fuchs & Crawford, 2003). Indeed, individuals who suffer from TBI usually display abnormalities in social and emotional functioning, such as impaired social competence (Marsh & Knight, 1991; Galski, Tompkins & Johnston, 1998; Janusz, Kirkwood, Yeates & Taylor, 2002; McGann & Werven, 1995), difficulties in understanding non-literal language and pragmatic communication(Bara, Cutica & Tirassa, 2001; Channon, Pellijeff & Rule, 2005; Dennis & Barnes, 1990; 2001; Dennis, Purvis, Barnes, Wilkinson & Winner, 2001; Turkstra, McDonald& Kaufmann, 1996; McDonald, Flanagan, Rollins & Kinch, 2003), impaired emotion recognition (Allerdings & Alfano, 2006; Jackson & Moffat, 1987), lack of empathy (Shamay-Tsoory, Tomer, Berger & Aharon-Peretz, 2003), egocentrism and inappropriate levels of social interaction(McDonald & Pearce, 1996), and difficulty applying social knowledge (Dimitrov et al., 1996).
A recent review by Martín-Rodríguez and León-Carrión (2010) found that, globally considered, TBI patients show a moderate to severe impairment in ToM. However, it is still matter of some debate whether the patients’ impaired ToM depends upon a primary and selective deficit in ToM abilities (e.g., Rowe, Bullock, Polkey & Morris, 2001), or is due to a deficit in a general cognitive ability, such as working memory (e.g., Davis & Pratt, 1995; Stone, Baron-Cohen & Knight, 1998), executive functions (e.g., Bora, Vahip, Gonul et al., 2005; Channon & Crawford, 2000; Hughes & Russell, 1993) or the ability to make general (non-mental) inferences (for a discussion, see Bibby & McDonald, 2005). Several studies have suggested the independence of executive dysfunctions and ToM impairments (e.g., Bach et al., 2000; Lough & Hodges, 2002; Havet-Thomassin et al., 2006), whereas others have demonstrated that these are associated (e.g., Henry et al., 2006; Rowe et al., 2001), although without finding any evidence of a causal relationship. These inconsistencies among findings may be due to the fact that most studies do not include control tasks for ToM tests, i.e., tasks aimed at assessing patients’ ability to draw non-mental inferences. A study by Bibby and McDonalds (2005) aimed to make good this lack, and controlled both for non-mental inferences and for the presence of working memory impairments. Their results showed that although TBI patients display a general weakness in inference-making, this does not fully account for the observed ToM deficits. More important, the inconsistencies among findings on ToM impairment in TBI patients may be due to the fact that most studies (such as Bach et al., 2006; Henry et al., 2006; Milders et al., 2003) measure ToM with respect to a single type of content, and then generalize their findings to the entire ToM ability. If, as we assume, ToM has a multicomponential nature, then such measures may not correctly capture the phenomenon, enhancing one component to the detriment of the others. To our knowledge, only one study, by Havet-Thomassin et al. (2006), has investigated two components of ToM: the ability to understand another individual’s emotional mental state from his/her eyes (assessed through the “Reading the Mind in the Eyes Test-revised version”, by BaronCohen, Wheelwright, Hill et al., 2001), and the ability to attribute intentions to another individual (assessed through the “Character Intention Task” by Sarfati et al, 1997). However, as the aim of the study was not to compare ToM ability when exerted on different types of content, the authors did not provide a comparative analysis of patients’ performance on the two assessment measures. As we detailed in Section 1, on the basis of the data from developmental psychology and neuroimaging studies, we assumed that ToM ability is multicomponential, and that such multicomponentiality depends upon the content of mentalization, that is the type of mental state (i.e., intention, emotion, belief) upon which the individual is reasoning.
In the present study we aimed to test our assumption by comparing the ability of TBI patients to make inferences about others’ beliefs, emotions and intentions. We expected to find a different degree of impairment in ToM tasks involving the understanding of these different types of mental states. As the type of lesion characterizing TBI patients is not selective, but typically diffused throughout the brain, we did not expect a selective deficit in a sub-component of ToM ability. Rather, we expected that all ToM components may be compromised, possibly with different degrees of impairment. Specifically, we hypothesized a possible trend of difficulty for the three types of mental states we examined. Indeed, the above cited studies on developmental psychology suggested that the ability to understand other people’s mental states ranges in complexity from the relatively simple understanding of desires and intentions, to the understanding of emotions as mental states, to the more complicated understanding of other people’s false beliefs (see also Flavell, 1999). Thus, we hypothesized that the task of understanding another person’s intentions is easier than the task of understanding another person’s emotions, which is in turn easier than understanding another person’s false beliefs.
To test the hypothesis that TBI patients exhibit different degrees of impairment in the understanding of other people’s intentions, emotions and beliefs, we created a protocol which distinctly assessed, in a comparable format, the abilities to understand false beliefs, intentions and emotions of other individuals (we called it the “fBEIC protocol”).
Furthermore, we administered a set of neuropsychological tests to all patients in order to assess those cognitive abilities, such as attention, memory, language and executive functions, in which a deficit could interfere with performance on mentalistic tasks. However, since ToM is a sophisticated activity that reflects high-level cognitive competence, while neuropsychological tests are typically meant to examine specific, low-level functions, it is unlikely that the performance on a single cognitive ability may predict the performance on a test of ToM. There is some evidence that attention and memory play a role in the classical false belief tasks, such as the Sally and Anne task (Baron-Cohen et al., 1985). Indeed, to solve it, the participant has to follow the actions of two characters in a narrative, and has to remember many details of the story (e.g., different positions of objects in time). These is evidence that, by reducing the memory and attention demand of the false belief task, the age at which children can solve the task decreases (see for instance Bloom and German, 2000). However, as in the protocol we reduced the attention and memory demands of the mentalistic and control tasks by employing drawings of the major passages of each story (see Material and Methods sections), we have no reason to predict a correlation between patients performance on the fBEIC protocol and that on neuropsychological tests evaluating attention and memory. Similarly, we have no reason to predict a correlation between patients performance on the fBEIC protocol and that on neuropsychological tests evaluating language abilities, as all the fBEIC stories were also represented through drawings. We also included some neuropsychological tests on executive functions, in reason of the long-standing debate about whether ToM is a distinct function or whether it is a part of executive processes. We know that developmental studies have demonstrated a link between the development of ToM and executive function abilities, both in typically developing and autistic children (e.g., Carlson, Moses, Brenton, 2002; Carlson, Mandell & Williams, 2004; Hughes, Russell & Robbins, 1994; Sabbagh, Xu, Carlson, Moses & Lee, 2006). Also, neuroimaging studies indicate that the prefrontal cortex is involved in both ToM and executive functioning (e.g., Sabbagh, 2004; Stone, Baron-Cohen & Knight, 1998; Stuss, Gallup & Alexander, 2001; Young, Dodell-Feder & Saxe, 2010). However, several other studies failed to find a relationship between executive functioning and ToM. Studies with older adults, for instance, showed that the decline of executive functioning does not correspond to a decline in ToM ability (e.g., MacPherson, Phillips & Della Sala, 2002; Maylor, Moulson, Muncer & Taylor, 2002; Happe, Winner & Brownell, 1998). Also studies with patients have shown conflicting results: for instance, Channon and Crawford (2000) found in adults with damage to the frontal lobes a correlation between deficits in executive functioning and deficits in ToM ability, but other researchers have failed to find a correlation (e.g., Lough et al., 2001; Rowe et al., 2001; Varley et al., 2001). For instance, Fine, Lumsden & Blair (2001) described a patient with a very severe impairment in ToM but no impairment in executive functioning, and Bird, Castelli, Malik, Frith and Husain (2004) described a patient with preserved ToM skills in the presence of an executive functioning impairment.
Clearly, the relationship between ToM performance and executive functioning remains controversial; for this reason, we have no predictions on the presence of a correlation between the performance on the fBEIC protocol and the performance to the executive function tests.
>
Neuropsychological assessment
The patients were presented with a set of neuropsychological tests (Italian validation: Spinnler & Tognoni, 1987) for the following functions:
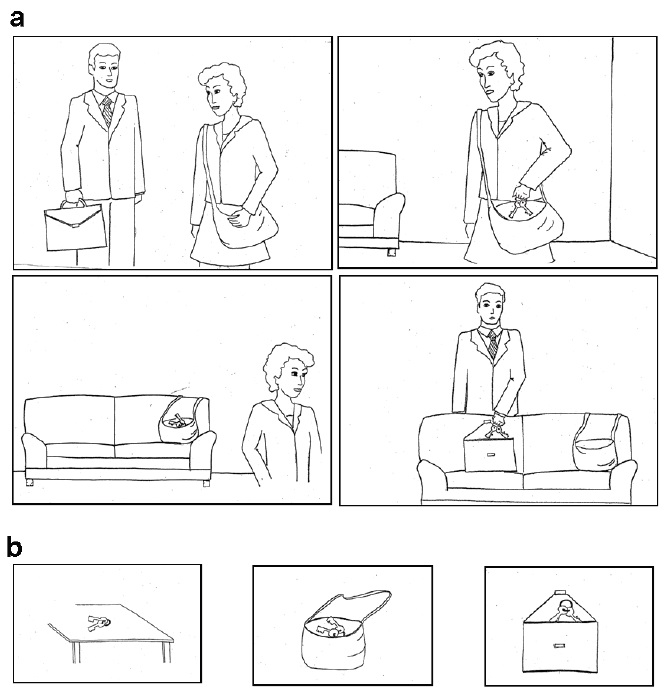
The protocol comprised 32 short stories. Each story consisted of a brief description of events, articulated in four passages. To reduce the memory and linguistic demands of the task, each passage was illustrated with a simple drawing (each printed on a white sheet of A5 paper) reproducing the current situation (see Figure 1a). We generated eight such stories for each of the three mentalistic abilities we investigated, and eight control stories. More in detail, the mentalistic stories included the following:
Each mentalistic story ended with a question on the main character’s state of mind; the question was in a forced-choice answer format, with three different answer-options (only one being correct). The three possible answers were illustrated in the form of a comic strip, each one printed on a sheet of A6 paper (see Figure 1b). As an example, the mentalistic question for the story in [1] was:
The protocol also comprised eight control stories, that requires participants to answer a question about the physical or behavioural consequences of an action or an event. Such kind of stories has often been used as control stories for mentalistic tasks (e.g., Baron-Cohen, Leslie & Frith, 1986; Brunet et al., 2003; Brunet, Sarfati, Hardy-Baylé & Decety, 2003a; 2003b). Notwithstanding such stories has been classically considered as non-mentalistic, because they do not require mental states attributions to be correctly resolved, however several recent studies on implicit mentalising (e.g., Apperly & Butterfill, 2009; German & Cohen, 2012; Qureshi, Apperly & Samson, 2010) prompt a clarification on this issue. Indeed, such studies show that an implicit mentalising automatically arises in response to stories involving characters: individuals usually process such stories spontaneously tracking characters’ mental states (e.g., Apperly et al., 2006; see German & Cohen, 2012 for a review). Also, an on-line processing of beliefs in response to tasks that do not require to answer any mentalising question has been highlighted by several neuropsychological studies (e.g., van Overwalle & Vandekerckhove, 2013). Therefore, we have to assume that our control stories, as well, may activate an automatic mentalising processing. Also, we can’t exclude that such implicit mentalising might affect the inferences drawn on the physical or behavioural outcomes of such stories. However, to the aim of this work, that is focused on explicit mentalising, we still consider this kind of stories as control tasks with respect to the explicit mentalising required to solve our mentalistic tasks.
The control stories in our protocol were similar to the mentalistic conditions 2 in terms of their overall structure, the number and the length of the passages and quality of language. They included the following story types, in which the participants had to understand, respectively, the characters’ behavior and physical actions:
The control stories were also followed by a question about the consequences of the actions and events; the question was in a forced-choice answer format, with three different answer-options (only one being correct). All the answer-options were illustrated in the form of comic strips, each printed on a sheet of A6 paper.
In addition, we generated four supplementary stories (one fB story, one E story, one I story and one BE story), that we used to introduce the participants to the task and train them on it.
A full description of the 32 stories included in the fBEIC protocol can be found in Appendix 1.
2Through all the paper, when referring to our protocol, with the terms “mentalistic story”, or “mentalistic item” or “mentalistic task”, we refer to “explicit mentalistic story”, “explicit mentalistic item”, “explicit mentalistic task”.
The participants dealt with the experiment individually, in a quiet room. They were told that the experiment was concerned with story comprehension. For patients the procedure consisted of two parts; the first was the
In the
At the beginning of the second session the participants were trained for the experimental task. To this aim we used four stories, similar in all aspects to those of the fBEIC protocol. The experimenter explained the task, telling the participants that they would be asked to listen carefully to a number of short stories accompanied by some pictures representing the main events narrated in the story, and then they would be asked a simple question.
Then the experimenter read the first story aloud while placing the drawings reproducing the main events in front of the participant. At the end of the story, the mentalistic (or the physical or behavioural) question was asked; the three alternative answers and the respective drawings were presented in random order. To reduce the memory demand of the task, the drawings representing the story remained visible while the test questions were asked. If participants were unable to make a choice or admitted they did not understand, the experimenter helped them to find the solution, explaining the task again and having them notice and correct the mistakes they might have made. When the participants appeared to have understood the task satisfactorily, the other training scenes were shown. Participants who were able to solve at least two of the four stories with no further help (or with self-repaired mistakes), were then submitted to the experimental protocol. None of the participants failed more than two of the four warm-up trials. Immediately after the training session the participants were presented with the real experiment.
The procedure was the same as for the training session. Stories belonging to the fBEIC protocol were presented in a random order, designed so that two instances of the same condition never occurred in consecutive trials. During the administration of the task a single five-minute break was inserted half way through the protocol (after the seventeenth story); however, the experimenter could include more breaks if asked to do so by the participant. The fBEIC session lasted approximately 35 minutes to one hour for patients and 20 to 40 minutes for controls. Each session was audiorecorded, after obtaining the participants’ informed consent.
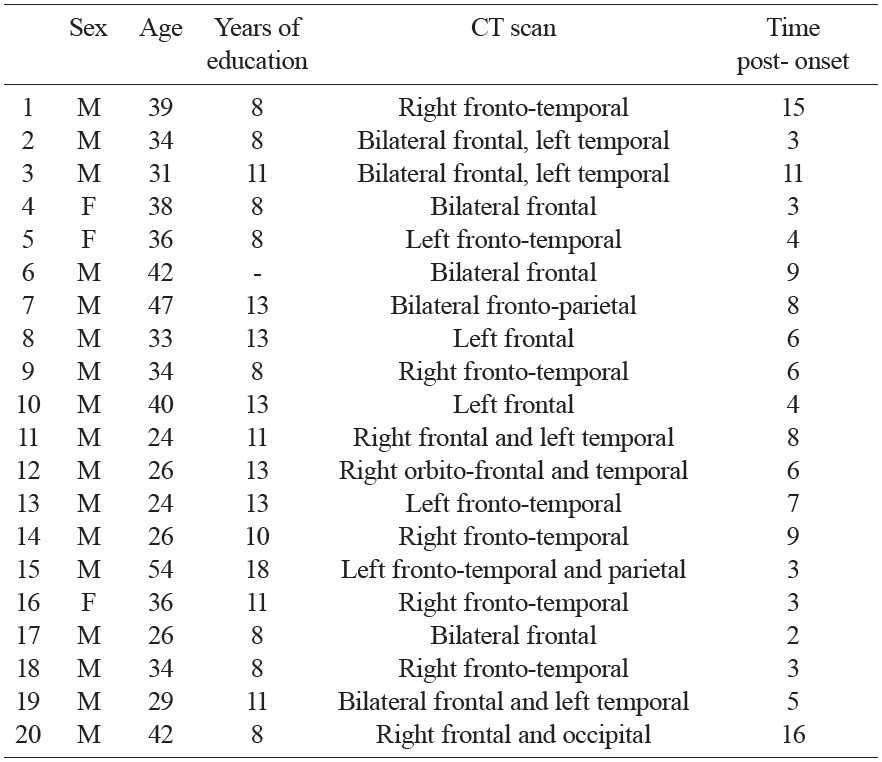
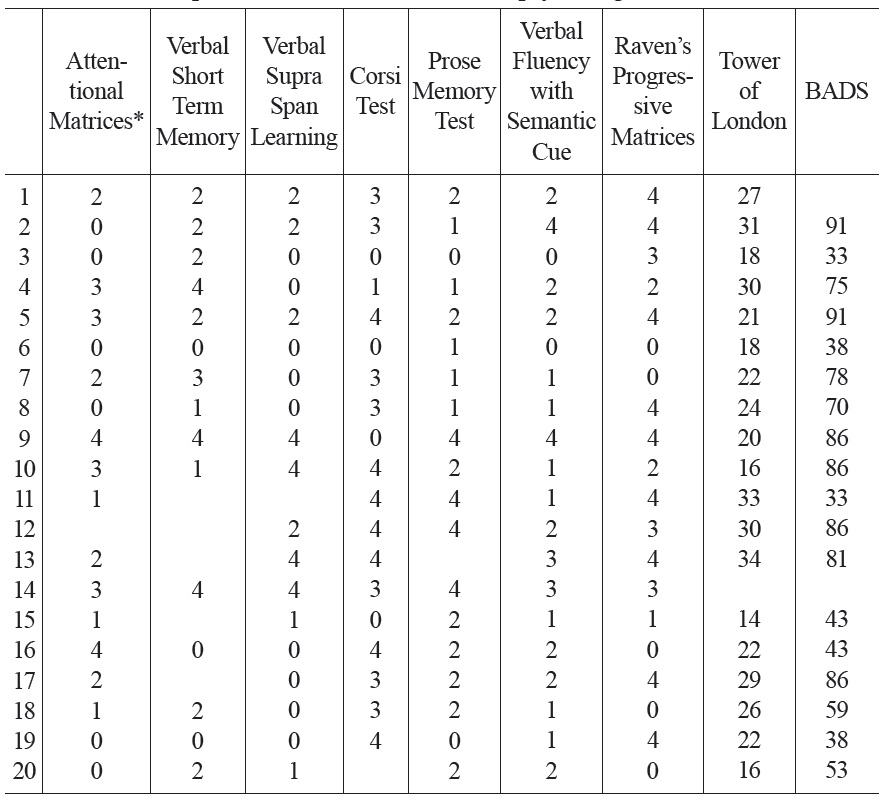
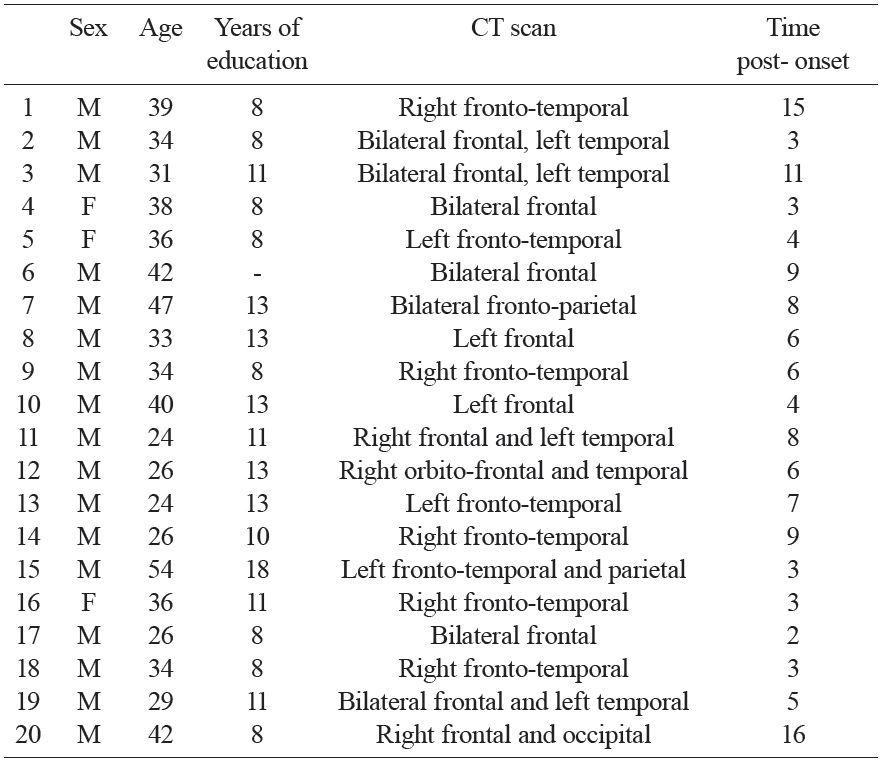
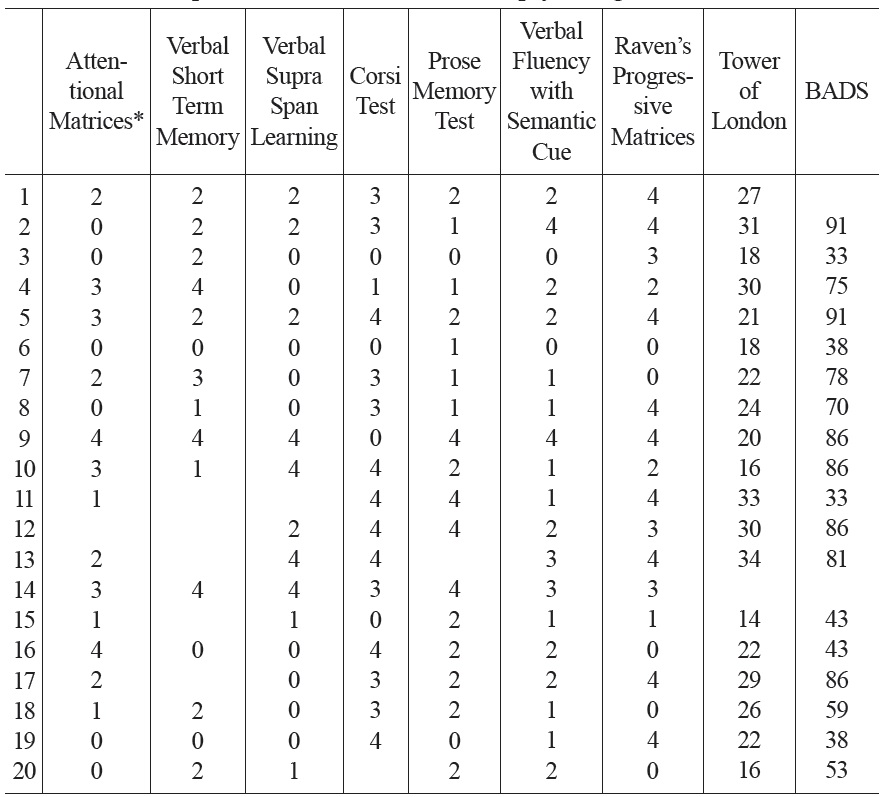
The patient group consisted of 20 TBI patients (17 males, 3 females), aged between 24 and 54 years (mean age: 34.7), and with between 8 and 18 years of education (mean years: 10.6). The patients were recruited from the Puzzle Social Cooperative in Torino (Italy), to which they had been admitted for postacute neuropsychological and motor, rehabilitation. All participants had suffered a head injury, most due to a motor vehicle accident. Minimum coma duration was one day, and the Glasgow Coma Scale scores (GCS), available for 12 patients, ranged from 3 to 14 (M = 5.6, SD = 3.2). All TBI patients had cerebral lesions shown on CT scan, in addition to diffuse axonal injury. An important inclusion criterion was the presence of lesions to the frontal lobes. Indeed, frontal lesions are known to be associated with ToM impairments (e.g., Rowe et al., 2001; Stuss et al., 2001), and, accordingly, TBI patients with frontal lesions are usually impaired in ToM (see Bibby & McDonald, 2005). At the time of the assessment, all patients were out of Post Traumatic Amnesia (PTA), and the time post-injury ranged from 2 to 16 years (M = 6.5, SD = 4.0). The clinical features of the TBI patients taken from medical records, and their demographic features, are reported in Table 1. None of the participants had deep linguistic comprehension deficits (evaluated through clinical assessment), or problems with their eyesight or hearing. The patients’ performance on standard neuropsychological tests for attention, memory, language and frontal functions is reported in Table 2.
[Table 1.] Demographic, neurological and neuroimaging data of TBI patients.
Demographic, neurological and neuroimaging data of TBI patients.
[Table 2.] Patients’ performance on standard neuropsychological tests.
Patients’ performance on standard neuropsychological tests.
The control group consisted of 20 healthy volunteers (male 15, female 5) matched on the basis of age, education and gender. The participants had no brain injury or history of neurological disorders. They ranged from 24 to 53 years of age (
Before analyzing performance for the fBEIC protocol we performed non-parametric analyses of variance using the Friedman test to verify an implicit assumption of our study, namely that participants would experience the same degree of ease/difficulty in understanding the eight stimuli that constituted each category (e.g. the eight intention condition stories). The results revealed that all the stories of a given sort were comparable in difficulty both for TBI patients (p value ranging from .115 to .529), and for controls (p value ranging from .097 to .368).
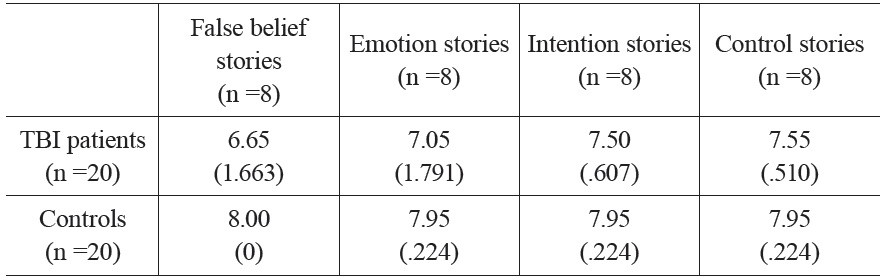
Results for both groups are reported in Table 3. In order to compare the performance of patients and controls in the fBEIC protocol, accuracy scores were submitted to between-subject ANOVA, with task (false belief, emotion, intention and control stories) as within-subject factor. Additional independent-sample T-tests were performed in order to compare the performance of patients and controls in the different tasks. To prevent multiple statistical contrasts from increasing the risk of obtaining false positives, we applied the Bonferroni correction. Results revealed that, globally, the performance of patients was significantly worse than the performance of controls (F(1,38) = 15.20, p < .001). No significant main effect of task was found (F(3,36) = 1.54, p = .220).
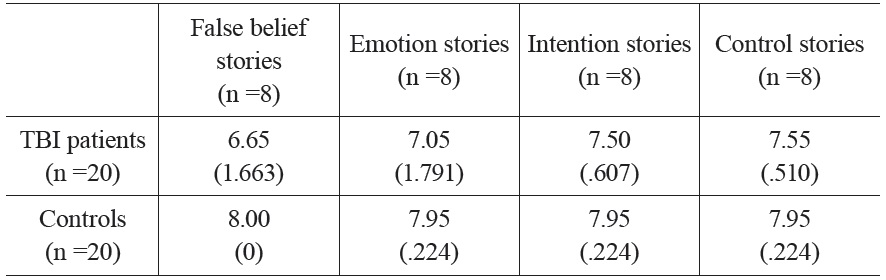
Mean correct performance by TBI patients and controls on the fBEIC protocol. Standard deviations are in parentheses.
The TBI patient group and the control group performed differently in the false belief stories task (t(38) = -3.63, p = .004), in the intention stories task (t(38) = -3.11, p = .016), and in the control stories task (t(38) = -3.21, p = .012). Although their performance in the emotion stories task differed in the direction we assumed, the difference was not statistically significant (t(38) = -2.23, p = .128).
Controls
The control group achieved almost the same degree of accuracy in the three categories of mentalistic stories of the fBEIC protocol (withinsubjects ANOVA, F(1,19) = 1.00, p = .330). No difference between mean performance in the mentalistic items and performance in the control items was found (paired-sample T-test, t(18) = -1.00, p = .330). Performance did not significantly differ from 8 (i.e, the maximum score) in any of the experimental conditions (t(18) = -1.00, p = .330 for all conditions), thus suggesting the presence of a ceiling effect for all conditions.
Patients
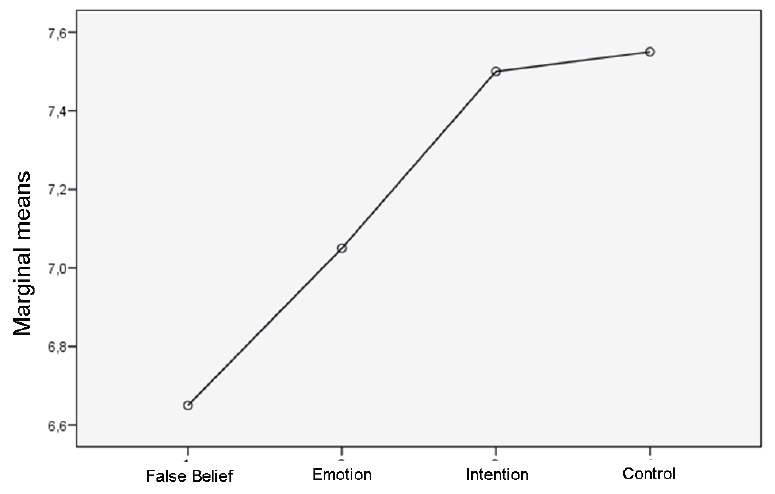
We tested the hypothesized trend of difficulty for the three mentalistic story categories, by applying the within-subjects ANOVA with linear contrast. Our results showed the following trend of increasing difficulty: intention items, emotion items, false belief items (F(1,19) = 5.43, p = .031). This trend is shown in Figure 2.
Mean performance in the mentalistic items was lower compared to performance in the control items, but the difference just approached statistical significance (paired-sample T-test, t(18) = 1.76, p = .094), mainly due to the high performance in the intention items. Considering the different mentalistic categories separately, performance in the false belief items was significantly lower compared to performance in the control items (t(18) = -2.27, p = .035). The difference between emotion items and control items was in the same direction, but did not reach statistical significance (t(18) = -1.21, p = .242). No significant difference between intention items and control items was found (t(18) = -.37, p = .716).
We found no correlation between patients’ performance on the fBEIC protocol and on neuropsychological tests evaluating attention, memory and linguistic abilities, either when considering each mentalistic category separately (Pearson correlation coefficient comprised between -.316 and .244, p-value between .188 and .941) or when collapsing the three mentalistic categories into a single variable (Pearson correlation coefficient comprised between -.243 and .093, p-value between .315 and .954).
Nor did we find any correlation between the fBEIC protocol and the neuropsychological test evaluating frontal functions and executive functions, either when considering each mentalistic category separately (Pearson correlation coefficient comprised between -.242 and .245, p-value between .154 and .997) or when collapsing the three mentalistic categories into a single variable (Pearson correlation coefficient comprised between -.144 and .058, p-value between .544 and .814). Also, no correlations were found between the neuropsychological tests and the control stories in the fBEIC protocol (Pearson correlation coefficient comprised between -.150 and .273, p-value between .245 and .935).
A main assumption of this study was that ToM is a multicomponential ability; we derived this assumption from several findings in developmental psychology and neuropsychological and neuroimaging studies. From this assumption, we hypothesized that ToM ability may comprise at least three different sub-abilities, as a function of the type of mental state that is the object of the meta-representation, i.e. emotion, intention, and false belief. Furthermore, as a consequence of the type of deficit that characterizes TBI patients, and of the developmental trend existing in children, we hypothesized that patients would find easier reasoning about others’ intentions compared to others’ emotions, and reasoning about others’ emotion compared to others’ false beliefs.
As a general result, the TBI group performed worse than controls on both mentalistic and control items, with the exception of emotion stories. The different performance in control items by the two groups is consistent with other studies in literature that claim that TBI does not result in a specific TOM impairment: patients with TBI seem to have a general weakness in inference-making, that impairs any kind of inference they should do.
In constructing the protocol we decided to use, for each item category, stories that were the simplest possible form of the corresponding ToM subcategory. As a consequence, we created very simple items, and most of the healthy adults in the control group solved all the items without difficulty, reaching a ceiling effect. Results showed that their performance was similar in all the tasks examined.
As concerning the TBI group, we did not find significant difference between their performance on control and mentalistic items (considered as a whole). This result is mainly due to the fact that the patients’ performance in the intention items was very similar to their performance in control items. Considering the different mentalistic categories separately, however, also the comparison between performance on emotion items and control items, albeit in the predicted direction, did not reach statistical significance. The only significant difference was between false belief items (that require to make inferences on two conflicting representations) and control items. Taken together, the present results add to previous findings (e.g., Bibby & McDonald, 2005) showing that general inference making is impaired in TBI patients, thus compromising their performance on both mentalistic and control tasks. However, it should be noted that a generic weakness in inference making should not be taken as evidence that ToM deficits in the TBI group are completely accounted for by inference making deficits (see Bibby & McDonald, 2005 for a discussion). Indeed, we found a different degree of impairment in different ToM tasks, depending upon the inferential content. In our view the most interesting result of the present study is that participants in the TBI group experienced a different degree of difficulty in dealing with different categories of mentalistic stories: results showed the following trend of increasing difficulty: intention items, emotion items, false belief items.
This result may account for some of the inconsistencies that have emerged in the literature on ToM ability in TBI patients: the different degree of impairment shown by patients may indeed explain the contrasting conclusions of studies that took into account inferences on just one type of mental state. More in detail, our results suggest that mentalistic tasks concerning false beliefs may not be good representatives of ToM ability, at least as long we are interested in ToM as the generic ability to attribute a mental state to another individual.
A further result of the present study is that - in the TBI sample – there was no correlation between ToM (as measured through the fBEIC protocol) and the neuropsychological tests evaluating frontal functions and executive functions. However, the aim of our study was not to evaluate whether TBI patients have a specific ToM deficit, and the neuropsychological tests we chose does not measure the full spectrum of human executive functioning.
In our view, in future research it would be also interesting to present the same protocol to children of different ages, in order to investigate any differences in the development of the ability to reason about others’ intentions, emotions and false beliefs.