Classical Ayurvedic texts are the oldest known ones pointing out that overweight, obesity, and diabetes are closely related lifestyle disorders, and that appropriate food choices and eating habits are necessary not only for proper maintenance of physical and mental health, but also for obtaining optimal therapeutic benefits from medicines and other health care measures (Rastogi, 2014; Sharma and Chandola, 2011a; Sharma and Chandola, 2011b). Modern nutritional and other researchers have now also well recognized that diverse phytochemicals commonly consumed with everyday meals have beneficial effects against overweight or malnutrition associated metabolic disorders encountered in patients suffering from, or at risk to diabetes, hyperlipidemia, and numerous others chronic diseases (Dembinska-Kiec et al., 2008; Farooqui, 2013; Gonzalez-Castejon and Rodriguez-Casado, 2011; Manach et al., 2009). Consequently, regular consumption of fruits, vegetables and other plant derived products are now highly recommended for reducing the risks of almost all such life threatening and silently progressing diseases (Anonymous, 2003; Agrawal et al., 2014). Despite extensive efforts and considerable progress (Janero, 2014; Kar and Roy, 2012; Singh et al., 2014a), prevention and cure of such disorders still continue to be a major challenge for both traditionally known as well as modern systems of medicine
Epidemiological, preclinical, and some clinical evidences now available on numerous edible plants strongly suggest though, that their modulating effects on psycho-biological processes involved pathogenesis and progression of lifestyle associated medical conditions are involved in their traditionally known health benefits (Dembinska-Kiec et al., 2008; Gonzalez-Castejon and Rodriguez-Casado, 2011; Baboota et al., 2013; Chandrasekaran et al., 2012; Chang et al., 2013a; Tiwari and Rao, 2002). It is now also well recognised that structurally and functionally diverse secondary plant metabolites are biosynthesized by them for defending themselves against environmental stress (Kwon et al., 2009), and that their traditionally known health benefits are most probably due to their modulating effects on environmental and metabolic stress triggered psychobiological processes involved in pathogenesis and progression of chronic diseases (Calabrese et al., 2012; Kennedy, 2014a). Dysregulation of these processes often leads to diverse spectrums of pathologies including obesity and diabetes and other silently progressing metabolic and inflammatory disorders (Cnop et al., 2012; Ozcan et al., 2004; Zhao and Ackerman, 2006). Amongst them, diabesity, i.e. obesity triggered type-2 diabetes, is the most rapidly spreading global epidemic of the 21st century now affecting all countries irrespective of their socioeconomic status and cultural background (Farag and Gaballa, 2011). The only currently available interventions with curative potentials against diabesity are bariatric surgeries (Kahn et al., 2014; Tschop and DiMarchi, 2012), and it is now well recognized that dietary therapies in combination with some antidiabetic or anti-hyperglycemic drugs (especially metformin) and physical exercise are the most effective means for preventing progression of diabesity associated physical and mental health problems (Colagiuri, 2010; Pratley and Matfin, 2007). However, due to socioeconomic, cultural, and diverse other reasons, such therapeutic possibilities and recommendations are either not available, or are not affordable and acceptable, in many economically developing and underdeveloped countries where the burden of diabesity is the utmost (Pan et al., 1997; Ramachandran et al., 2006; Ramachandran et al., 2012).
Culinary uses of numerous edible plants used in Ayurvedic and other traditionally system of medicine and health care are very popular in all such countries, and irrespective of their socioeconomic and cultural backgrounds, herbal therapies are the most affordable and acceptable health care options for a vast majority of population in most of them. Therefore efforts are now being made in many laboratories to identify edible and other plants with anti-hyperglycemic, anti-inflammatory, insulin sensitivity improving and other diabesity associated pathologies (Eddouks et al., 2014; Chang et al., 2013b; Leiherer et al., 2013). A consistent observation made with diverse types of extracts of numerous such edible and other plants in animal behavioral models has been that their stress response regulating and other efficacies increase with increasing number of days of treatments (Chatterjee and Kumar, 2012; Kumar and Chatterjee, 2014a). These and numerous other observations made during efforts to define pharmacological activity profiles of several such extracts strongly suggest that biological mechanisms and processes involved in antihyperglycemic or anti-diabetic efficacies of edible plants could as well be due to their stress response modulating effects, and that structurally diverse secondary plant metabolites ubiquitously encountered in many, if not most, edible and other plants are also involved in their such efficacies (Langstieh et al., 2014; Shivavedi et al., 2014a; Shivavedi et al., 2014b; Rauniyar et al., 2015; Verma et al., 2015; Shakya et al., 2015). In this communication, available preclinical and clinical information on a few Ayurvedic edible plants justifying these inferences are summarized and critically analyzed and discussed in light of our current understanding on stress response regulating potentials of edible and other phytochemicals.
Brassica juncea (Mustard)
Commonly known as oriental or brown or Indian mustard, Brassica juncea L. is one of the numerous edible plants of the Brassicaceae family now widely used in India and other countries for obtaining mustard seeds and edible oil. It is a draught resistant plant (also often considered as weed by agricultural industries) widely cultivated for meeting the great commercial demand of mustard seeds and oil with nutty taste and pungent aroma. Its green leaves (commonly called mustard green) are also often used as spicy vegetables, salad, and pickled condiment in many Asiatic countries. Medicinal uses of diverse varieties of mustard plants are mentioned in classical Ayurvedic texts, and Brassica juncea is one of the several plants of the family mentioned in such texts (Manohar et al., 2009). Nutritive values of mustard green and diverse medicinal and health care potentials of mustard seeds and oils have been known since long not only in India, but also in numerous other countries. Although nutritive values of edible green leaves of Brassica juncea have also been mentioned in classical Ayurvedic texts, they are seldom used in Ayurvedic pharmaceutical formulations.
Information now available on medicinal phytochemistry of Brassica juncea leaves reveal though, that numerous bioactive phytochemicals encountered in them are either structurally identical or functionally similar to those identified in not only in different varieties mustard seeds, but also in many other pharmacologically and clinically better scrutinized edible plants of the Brassicaceae family. Amongst oil producing plants of this family, Brassica juncea is currently one of the major mustard oil producing crops in India, which is one of the major vegetable oils commonly consumed in the country (Mishra and Manchanda, 2012; Singh et al., 2014b). It has been suggested that regular consumption of mustard oil together with vegetarian diet could be a feasible dietary means for lowering the risk of ischemic heart diseases in Indian population (Rastogi et al., 2004). Since bitter and pungent taste of mustard oil and all condeiments and vegetables prepared from plants of the Braceacea family are not always well accepted by many consumers (Drewnowski and Gomez-Carneros, 2000), they are often not regularly consumed, or are well accepted, for culinary purposess by many consumers. Therefore, efforts are now being made by food and agricultural industries to obtain Brassica juncea products devoid of such tastes (Sindhu et al., 2012).
Another reason for obtaining different cultivars of Brassica juncea is to obtain mustard oil with lower contents of Eruic acid, i.e an omega-9 fatty acids which has been reported to possess adverse health effects (Singh et al., 2013). Since potential adverse health effects of eucric acid (Sauer and Kramer, 1983; Choudhary et al., 2014), and also those of the pungent and bitter tasting isothiocyanate and other phytochemicals encountered in Brassica juncea derived products (Tripathi and Mishra, 2007; Inyang et al., 2014) have been observed in some animal bioassays, health care authorities of several countries have issued warnings against, or have even banned, edible uses of mustard oils (Oram et al., 2005; Wendlinger et al., 2014). However, as yet no very definitive statements based on preclinical, or clinical, or epidemiological evidence on maximally tolerated daily oral doses of such acids and numerous other phytochemicals commonly consumed with diverse types of mustard derived products can be made. This is mainly because appropriate dose response and other studies necessary for estimating their pharmacologically interesting dose ranges, safety profiles, and possible interactions between them are still missing.
On the other hand, the numbers of reports suggesting potential health benefits of diverse types of Brassica juncea leaf and seed extracts and their bioactive constituents have continued to increase during recent decades (Kumar et al., 2011a). Apart from vitamins, minerals, and nutritive proteins and lipids, the plant is now well recognized to be a rich source of a numerous phytochemicals often encountered in diverse other edible plants and well known for their cellular stress response modulating and hormetic effects (Birringer, 2011; Calabrese, 2010; Calabrese et al., 2010). However, since in Ayurvedic system of medicine mustard derived products are always used in combination with other natural products and health care procedures, therapeutic relevance of these findings in their traditionally known medicinal uses still remain questionable or speculative only.
The most extensively studied bitter and pungent tasting secondary metabolites encountered in Brassica juncea are the glucosinolates. Glucobrassicin, neoglucobrassicin, 4-methoxy glucobrassin and 4-hydroxy glucobrassicin are some indole glucosinolates more often encountered in Brassica juncea than in other plants of the family (Schreiner et al., 2009).
Quantitatively though, sinigrin is one of the major glucosinolate encountered in Indian mustard (Sang et al., 1984), which is now often considered to be a bioactive secondary metabolite of several edible plants with anti-cancer and anti-microbial activities (Patel et al., 2012). During processing of mustard seeds glucosinolates are enzymaticaly degraded to allyl-isothiocyanate, which possesses strong bactericidal activities. Therefore, mustard meal powders are often recommended as a natural antimicrobial agent (Munday and Munday, 2002; Dai and Lim, 2014). Structurally diverse glucosinolates are also well known for their preventive potentials against cancer and other chronic diseases, including diabetic neuropathy and neurodegenerative diseases (Halkier and Gershenzon, 2006; Fahey et al., 2003; Tarozzi et al., 2013; Dinkova-Kostova and Kostov, 2012)
Essential oils of Brassica juncea consists mainly of a group of structurally analogous isothiocyanates. Some of them, also found in edible mustard oil, are allyl isothiocyanate, diallyl trisulfide, 3-butenyl isothiocyanate, allyl isothiocyanate, diallyl trisulfide and 3-butenyl isothiocyanate (Yu et al., 2003). It has been estimated that such oils consists of ca. 11% saturated and 89% unsaturated fatty acid of which about 18% is linoleic and 15% is linolenic fatty acid (Mishra and Manchanda, 2012). Other fatty acids found in Brassica juncea are erucic, eicosanoic, arachidic, nonadecanoic, behenic, oleic and palmitic acids, and arachidonic and α-linolenic acids are also been encounter in its oils (Kumar et al., 2011a). Brassicasterol, campesterol, β-sitosterol, Δ5-avenasterol and trace amounts of Δ7-stigmasterol have also been isolated and characterized from the mustard seed oil (Li et al., 2000).
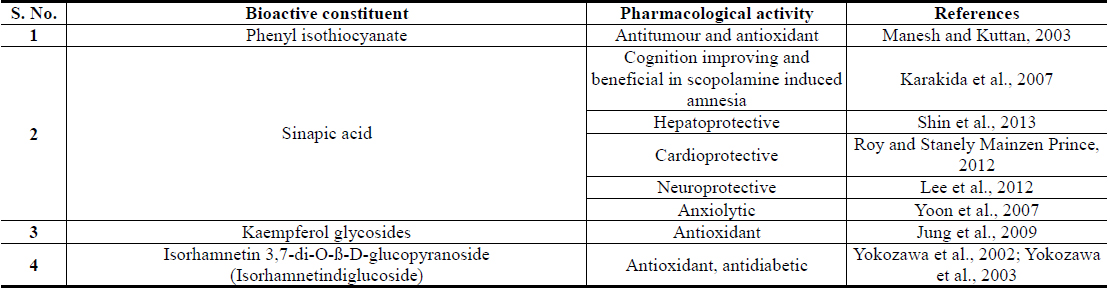
Plant polyphenolics encountered in Brassica juncea, and in numerous other edible plants now attracting major attention of modern nutritionists and herbal researchers (Wang et al., 2014; Xiao and Hogger, 2015; Pandey and Rizvi, 2009). These are structurally diverse polyphenolic acids, quercetin, kaempferol, isorhamnetin and their naturally occurring conjugates, derivatives, and analogues (Cartea et al., 2010; Kumar and Andy, 2012; Kumar et al., 2012). Sinapic acid and its conjugates are quantitatively the major polyphenolics of Brassica juncea and it has been reported that it is one of richest natural sources of the acid and its conjugates (Niciforovic and Abramovic, 2014). Like for diverse other polyphenolic of Brassica juncea leaves, sinapic acid has also been identified as an antidiabetic agent with stress response modulating and antidepressant, anxiolytic and other brain function modulating activities (Cherng et al., 2013; Yoon et al., 2007).
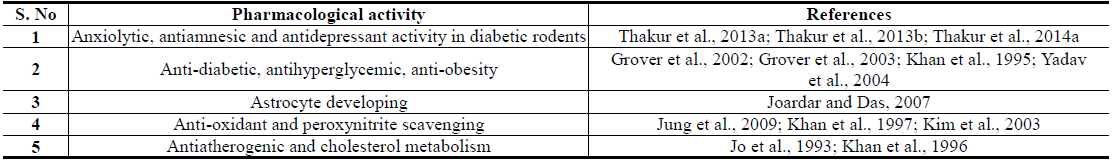
Isorhamnetin is also another Brassica juncea specific and quantitatively major flavonid of mustard green with an analogous broad spectrum of therapeutically interesting bioactivity profile. Although numerous flavonoid glycosides with oxidative stress protecting activities have been isolated from Brassica juncea Leaves (Jung et al., 2009; Kim et al., 2002), isorhamnetin glycosides have been reported to be their major antidiabetic component with such activities (Yokozawa et al., 2002; Yokozawa et al., 2003). A comparative study on flavonoid contents of 91 vegetables has revealed an unique flavonol aglycone spectrum in mustard green, and found that it has the highest amount isorhamnetin amongst all vegetables analyzed in that study (Yang et al., 2013). It must be mentioned though, that isorhamnetin and its conjugates are also human metabolites of quercetin and other naturally more abundant flavonoids (Manach et al., 2004). However, like for all other plant extracts, the pharmacological activity profiles, or antidiabetic activity, of Brassica juncea leaf extracts cannot be predicted from their contents of phenolic plant metabolites, or by their antioxidative potentials, only (Thakur et al., 2013a; Thakur et al., 2013b; Thakur et al., 2014a).
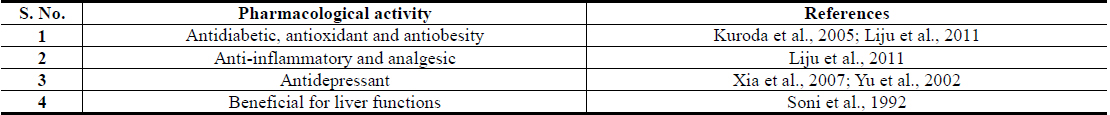
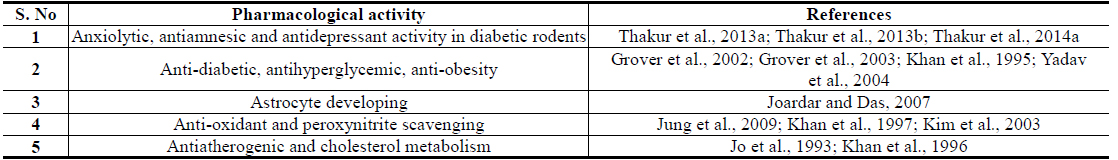
Information now available on preclinical pharmacology of diverse types of extracts, and some of their often discussed bioactive constituents, suggesting their preventive and curative potentials against obesity and diabetes associated comorbidities are summarized in Tables 1 and 2 respectively. These and numerous other therapeutic possibilities offered by Brassica juncea and its bioactive secondary metabolites strongly suggest that modulation or regulation of psychological and metabolic stress triggered biological processes involved in etiology, pathogenesis, and progression of diverse lifestyle associated medical conditions and accelerated aging (Dietrich and Horvath, 2012; Epel, 2009; Fontana, 2009) are involved in their modes of actions. Consequently, efforts are now being made in several laboratories, including ours, to identify stress response modulating secondary metabolites of the plant, necessary for obtaining more rationally standardized extracts of the plant suitable for further developments as phyto-pharmaceuticals, or nutraceuticals, against diabesity and other lifestyle and environmental stress triggered mental health problems.
For such purposes, or for dietary therapy with Brassica juncea derived food, it is necessary to cultivate, harvest and process the appropriate variety or strain of the plant. This is not only because the content of bioactive secondary metabolites of the plant vary considerably in different strains and cultivars of the plant, but also due to the fact that it is an accumulator of toxic metals like lead, arsenic, cadmium etc. (John et al., 2009; Podar et al., 2004; Jakovljevic et al., 2013). Blood levels of such metals in diabetic patients are often fairly high (Akinloye et al., 2013; Kuo et al., 2013; Afridi et al., 2008), and their adverse effect potentials are well known. This is another reason behind the efforts of agricultural and food industries, and researchers to identify Brassica juncea cultivar strains and cultivation conditions for increasing crop yields with lower heavy metal contents. Although some strains of the plant with high crop yields have been identified, many questions concerning their contents of healthy or unhealthy substances still remain to be solved.
Such questions can be more rationally answered by collaborative efforts of medicinal phytochemists, herbal pharmacologists and toxicologists only. Availability of a convenient and well validated bioassays for identifying not only the therapy relevant secondary metabolites of the plant, but also for quantifying potential synergistic, antagonistic, additive effects between them is an essential prerequisite for such purposes. Since numerous known bioactive secondary metabolites of Brassica juncea and almost all edible medicinal plants possess physiological stress response regulating activity (Barrajon-Catalan et al., 2014; Joven et al., 2013; Lee et al., 2014; Tomas-Menor et al., 2015), it now apparent that traditionally known medicinal uses of mustards and other edible plants derived products in Ayurvedic system of medicine and health care is mainly due the presence of specific combinations of such substances in Ayurvedic formulations or in Ayurvedic vegetarian food menus. Therefore, it can safely be suggested that the now well validated mouse bioassay evolving from efforts to identify and pharmacologically characterize the stress response regulating constituents of Brassica juncea and other edible plants (Langstieh et al., 2014) could be an useful one not only for better understanding of Ayurvedic pharmacology, but also for discovering and developing urgently needed novel preventive and curative pharmaco-therapies against diabesity and other lifestyle associated physical and mental health problems.
Piper longum
Piper longum L., commonly known as Pippali or “Indian long pepper”, is a wildly growing and flowering perennial climber belonging to edible plants of Piperaceae family. It is now cultivated also for its fruits, which are usually dried and used as a spice and seasonings. Piper longum fruits have a similar, but somewhat hotter or more pungent taste than its close relative Piper nigrum, from which black, green and white pepper is obtained. The oldest known references of Piper longum come from ancient Ayurvedic texts where diverse medicinal and dietary uses of different parts of the plant are described (Manoj et al., 2004). In traditionally known Chinese system of medicine, the fruits are often used for treatments of hyperlipidemia, and during recent years, at least four structurally diverse anti-hyperlipidemic constituents, i.e. piperine, piperlongumine, pipernonaline, and 7, 4’-dimethyl ether of apigenin, have been identified from the fruits. According to these reports (Jin et al., 2009; Krishna et al., 2014), anti-hyperlipidemic efficacies of some of these Piper longum constituents in animal models are comparable to the currently widely used antihyperlipidemic drug simvastatin. However, the numbers of phytochemicals isolated from different parts of Piper longum, and their diverse bioactivities suggesting preventive and curative potentials of the plants against diverse malnutrition and other environmentally triggered inflammatory pathologies are not necessarily limited to these molecules only (Kumar et al., 2011b; Zaveri et al., 2010).
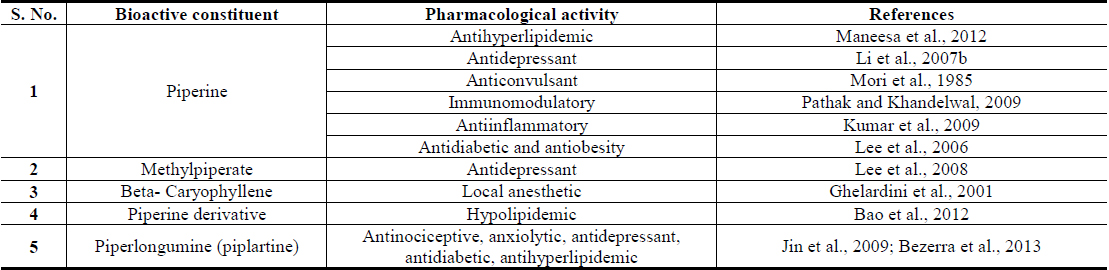
Reports on structurally and functionally novel secondary metabolites of the plant as well as their novel therapeutically interesting and other bioactivities, or of their since long known secondary metabolites, have continue to appear during recent years (Yang et al., 2013; Ahmed et al., 2014; Huang et al., 2010; Jiang et al., 2013; Ku et al., 2014; Yang et al., 2014, Yadav et al., 2014). Apart from proteins, carbohydrates, starch, and other nutritive constituents, and volatilile oils, most known bioactive molecules isolated from different parts of the plant are often structurally classified as alkaloids (alkaloidal amides), saponines, lignans, and phenolics etc. However, none of them are very specific biomarkers of the plant, and all of them have also been isolated from other edible plants of Piperacea and other families. Some of the numerous alkaloids or alkaloidal amides encontered in Piper longum fruits are: piperine, methyl piperine, iperonaline, piperettine, asarinine, pellitorine, piperun decalidine, piperlongumine, piperlonguminine, refractomide A, pregumidiene, brachystamide, brachystamide-A, brachystine, pipercide, piperderidine, longamide and tetrahydropiperine, terahydro piperlongumine, dehydropipernonaline piperidine, piperine, terahydropiperlongumine and trimethoxy cinnamoyl-piperidine and piperlongumine (Zaveri et al., 2010). Piperine is often considered to be quantitatively the major (ca. 3 - 5% on dry weight basis) bioactive constituent of long pepper and it is also the major pungent tasting secondary metabolite of the plant. Therefore, it is now often used as a biomarker for analytically standardizing Piper longum extracts for experimental as well as commercial purposes.
Pungency of piperine is caused by activation of the heat and acidity sensing ion channel TRPV1 on pain sensing nerve cells (McNamara et al., 2005). Such capsaicin like effects of piperine and other pungent alkaloidal amides of Piper longum are most probably also involved in pain response modulating effects of its diverse types of extracts. Altered pain sensitivity is a cardinal symptom of diabetic neuropathy and other inflammatory disorders, and crucial role of TRPV1 channel in etiology, pathogenesis, and progression of diabesity associated pathologies are now well recognized (Suri and Szallasi, 2008). However, the questions concerning the involvement of these ion channels in pain response regulating functions of brain or in observed mental function regulating effects of Piper longum and other herbal extracts, cannot yet be answered with certainty (Gunthorpe and Szallasi, 2008). It remains certain though, that piperine and other alkaloidal amides with pungent and spicy tastes are modulators of the functions of TPRV1 channel (Rios and Olivo, 2014) and that as a desensitizer of this channel piperine is more efficacious than capsaicin (Szallasi, 2005).
Another bioactivity of piperine now attracting major attention of modern researchers is its ability to enhance bioavailability of other nutrients and drugs (Patil et al., 2011). Similar or analogous effects are also known for numerous other edible phytochemicals commonly consumed with every day meals (Muttepawar et al., 2014; Tatiraju et al., 2013). Although as yet no very definitive statements on biological mechanisms and process involved in such effects of piperine can be made, it remains certain that its regular consumption can enhance oral bioavailability of other essential nutrients and edible phytochemicals commonly consumed with everyday meals, or with phyto-pharmaceuticals and nutraceuticals. It has been reported, indeed, that piperine enhances oral bioavailability of the edible antidiabetic phytochemical curcumin by 2000% in human (Shoba et al., 1998), which is the quantitatively major bioactive constituent of another Ayurvedic herb Curcuma longa (to be described later). These and numerous other analogous observations made with piperine and other edible phytochemicals strongly suggest that modulations of bioavailability of essential nutrients are also involved in their observed beneficial effects on metabolic disorders, and that proper understanding of their Ayurvedic pharmacology and medicinal values is possible only when due attention is paid to their such properties.
Another major alkaloidal amide encountered in Piper longum and attracting major attention of modern drug discoverers is piperlongumine, or piplartin (Bezerra et al., 2013). It is not encountered in Piper nigrum, the fruits of which are the most commonly consumed spice in the western world and elsewhere (Bezerra et al., 2013). Preclinical information now available on piplartin strongly suggest that it is most probably also one of the major bioactive secondary plant metabolites involved in diverse therapeutically interesting mental and metabolic function modulating effects of Piper longum fruits and roots (Bezerra et al., 2013), and that it could be a promising cancer therapeutic lead as well (Wu et al., 2014). Although piperlongumine and piperine are the two quantitatively the major bioactive constituents of numerous piper species commonly used in numerous Ayurvedic formulations (Meghwal and Goswami, 2013), their quantities vary considerable not only in different piper species but also in different parts of Piper longum and other plants of the species (Bao et al., 2014; Chandra et al., 2014).
Piplartin was first isolated from Piper longum roots, dried powder of which are also often used by Ayurvedic and other practitioners of herbal medicine for treatments of insomnia and debility caused by chronic fever (Murthy, 2009), and diverse types of extracts of the roots and their combinations with other herbal extracts are now also commercialized in India as Ayurvedic remedies (Rajopadhye et al., 2012). Such remedies are often used for treatment of rheumatism and other inflammatory conditions, and aqueous suspension of powdered roots of Piper longum root, commonly called Pippalimula in India, has been reported to possess ibuprofen like analgesic activity (Vedhanayaki et al., 2003). During recent years, several reports revealing antidiabetic activities of aqueous and ethanolic extracts of Piper longum roots have also appeared (Chaurasia and Das, 2013; Chaurasia et al., 2012; Nabi et al., 2013). Extensive psycho-pharmacological and some clinical observations made in our Banaras Hindu University’s Ayurvedic hospital with Piper longum root powder have not only revealed its broad spectrum of therapeutically interesting neuronal function modulating activities, but also indicated that metabolic as well as psychological stress response modulating effects are also involved in its modes of actions. Since stress response regulating properties of a Piper longum fruits containing Ayurvedic formulation has been reported (Neha and Mishra, 2011), and several bioactive phytochemicals encountered in the roots and fruits used in the formulation are the same, it could as well be that the reported antidiabetic activity of the root extracts are also due to the presence of stress response regulating components in them.
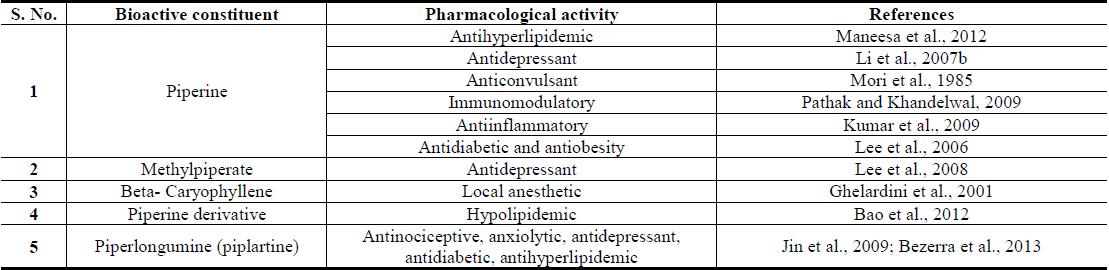
Major therapeutically interesting bioactivities of diverse types of Piper longum extracts and their quantitatively major bioactive constituents indicating therapeutic potentials of the plant for prevention and cure of obesity associated comorbidities are summarized in Tables 3 and 4 respectively. Although several recent reports have already revealed anti-obesity activity of piperine containing Piper longum oil in animal models (BrahmaNaidu et al., 2014; Choi et al., 2013; Doucette et al., 2013; Kumar et al., 2013; Noble et al., 2013), many questions concernig the roles of other bioactive constituents in such oils still remain open. Moreover, many such reports pay little attention to the fact that piperine and other constituents of such oils also possesses mental function regulating activities (Cicero Bezerra Felipe et al., 2007; Gilhotra and Dhingra, 2014; Li et al., 2007a; Li et al., 2007b; Mao et al., 2014; Pal et al., 2011), and that their modulating effects on central nervous system could as well be involved in anti-obesity and other health benefits of Piper longum derived products against type-2 diabetes (Sandoval et al., 2009; Patrone et al., 2014; Banks et al., 2012). It cannot be ignored though, that due to very hot taste of such products, they might not be well suited for incorporating them in everyday meals in high enough quantities necessary for obtaining health benefits from them.
Amlaki and Triphala
Amlaki is the Sanskrit name of the edible fruits of Phyllanthus emblica (synonym: Emblica officinalis; common name: Indian gooseberry) most commonly used in Ayurvedic system of medicine and health care as a tonic and for prevention and cure of physical as well as mental health problems arising from intricate disorders of digestive and excretory organs. It is also one of the most widely used edible fruits in numerous Ayurvedic formulations. One such very popular formulation is Triphala, which consists of equal parts of dried edible fruits of Embelica officinalis, Terminalia chebula, and Terminalia bellirica (Belapurkar et al., 2014; Maheshwari and Rajnee, 2014). Preventive and curative use of both Amlaki as well as of Triphala with barley and other dietary measures for treatments of diabetes is recommended in ancient Ayurvedic texts, and their such medicinal uses are now well justified by numerous preclinical and some therapeutic observations reported during recent decades (Sharma and Chandola, 2011a; Sharma and Chandola, 2011b; D’Souza et al., 2014; Mohammad and Larijani, 2013; Tiwari, 2008; Rajan and Antony, 2008). In classical Ayurvedic texts numerous other formulations containing them are mentioned also (Chulet and Pradhan, 2009; Chouhan et al., 2013), and in traditionally known Indian, Chinese, and Arabic systems of medicine Triphala is more often than not used in combination with other medicinal herbal herbs and other health care practices (Mohammad and Larijani, 2013; Zaki et al., 2014). Amongst the three edible fruits constituting Triphala formulations, the one most widely harvested and consumed for culinary purposes is Amlaki.
Recent neuropsychopharmacological observations (Kumar and Chatterjee, 2014b; Dhanalakshmi et al., 2007; Dhanalakshmi et al., 2006; Nariya et al., 2011; Rinki and Mishra, 2011; Srikumar et al., 2006) have revealed a broad spectrum of neuronal function modulating effects of Triphala, and suggest that its modulatory effects against glucose toxicity and diverse metabolic or mental stress triggered biological responses could be involved in its modes of actions. It is a sour, bitter, and astringent tasting fruit, which is often steeped in salt water and turmeric to make the sour fruits palatable. Gallic and Elegiac acids and their conjugates are some of the quantitatively major bioactive constituents of all the three fruits composing Triphala (Patel and Shah, 2009). However, the spectrum of known bioactive secondary plant metabolites encountered in Amlaki is not identical to those known for the other two fruits commonly used in this and numerous other Ayurvedic formulation. Available information on the bioactive constituents, and experimental evidences suggesting therapeutic potentials of Amlaki for treatments of diabesity and diverse other diseases have often been reviewed during recent years (D’Souza et al., 2014; Dasaroju and Gottumukkala, 2014; Khosla and Sharma, 2012; Krishnaveni and Mirunalini, 2010; Sri et al., 2013). Amlaki is also one of the several Ayurvedic medicinal herbs currently universally well recognized by modern herbalists and herbal researchers as an herbal adaptogen (Winston and Maimes, 2007). The concept of herbal adaptogens evolved originally in Russia during late 1940s (Brekhman and Dardymov, 1969), and the very first more systematic and detailed report on adaptogenic, or biological stress response regulating, effects of Amlaki and a few other edible and other Ayurvedic medicinal plant appeared in 1999 (Rege et al., 1999). Since then this concept has been well accepted by almost all modern scholars and practitioners of Ayurvedic system of medicine, and efforts are now being made by modern researchers to experimentally verify the efficacies of combinations of Amlaki with other edible plants and Ayurvedic therapeutic practices for prevention and cure of type-2 diabetes (Tripathi et al., 2012; Vaibhavi et al., 2013).
One of the several Amlaki formulation specially recommended in classical Ayurvedic texts for treatment of diabetes-associated comorbidities is Nishamlaki, which consists of Amlaki and Turmeric rhizome (to be described later). They are also the two major constituents of a vast majority of herbal formulations currently commonly prescribed in India for treatments of diabesity (Sarma et al., 2014). Therefore, efforts are now being made to better standardize such a formulation that could eventually be more rationally developed and used for prevention and cure of diabetes (Rao et al., 2013; Venkateshwarlu et al., 2013). A recent report have revealed though, that inappropriate formulations or doses of such preparations could as well have adverse drug-drug interaction with metformin (Puranik et al., 2014), i.e. one of the main anti-diabetic drugs commonly recommended for treatments of type-2 diabetes. Efforts to answer the question whether Amlaki, or Turmeric, or inappropriate combinations of the two are involved in such herb-drug interaction will be necessary for more rational uses of the two edibles for prevention and cure of diabesity. Moreover, since both Amlaki as well turmeric are often consumed in India with every day meals, and both diabetes and obesity are also the most common metabolic disorders encountered in Indian, China, and other developing countries, efforts to better clarify the situation is urgently needed for obtaining appropriate medicinal benefits from metformin and other antidiabetic drugs in these countries (Neerati et al., 2012).
Available information on medicinal phytochemistry of the other two fruits constituting Triphala strongly suggest that they could also have adverse herb-drug interactions with metformin and other drugs currently often prescribed for prevention and cure of diabesity associated physical and mental health problems (Fasinu et al., 2012). Although a few known bioactive secondary metabolites of Emblica officinalis (D’Souza et al., 2014; Khosla and Sharma, 2012; Zhang et al., 2001; Rastogi and Mehrotra, 1995; Asolkar et al., 1992; Dasaroju and Gottumukkala, 2014), are not encountered in Terminalia chebula (Rastogi and Mehrotra, 1995; Saleem et al., 2002; Kokate et al., 2003; Rathinamoorthy and Thilagavathi, 2014; Walia and Arora, 2013; Gupta, 2012) or in Terminalia bellirica (Saleem et al., 2002; Kokate et al., 2003; Kadian et al., 2014; Kumudhavalli et al., 2010; Saxena et al., 2013), all of them are fairly rich sources of tannins, gallic acids and their conjugates, and several other secondary plant metabolites ubiquitously present in many edible and other plants and well known for their modulating effects on drug metabolizing enzymes and their bioavailability (Serrano et al., 2009; Anannarukan et al., 2012). It has been reported that drug metabolizing enzyme inhibitory properties of Triphala are involved in such observed effects of the formulation, and that in this respect all the three constituents of the formulation are almost equipotent (Ponnusankar et al., 2011). According to this report, gallic acid and its conjugates and derivatives are also their common polyphenolic constituent involved in such effects of Triphala.
It must be mentioned though, that gallic acid conjugates and other plant polyphenolics are also encountered in numerous other edible and medicinal plants, and that they are now well recognized for their protective effects against oxidative stress triggered pathologies, including diabetes and associated psychopathologies (Lee et al., 2014; Dragan et al., 2015; Khadem and Marles, 2010; Lephart, 2015; Liu et al., 2015; Santilli et al., 2015). Moreover, analgesic, anti-inflammatory, antidiabetic, anxiolytic, antidepressant, cognitive function modulating, antimicrobial, and diverse other therapeutically interesting bioactivities of diverse types of extracts of Amlaki and Triphala enriched in such phytochemicals also have often been reported. Detailed discussions and critical analysis of these reports dealing with their diverse such bioactivities is beyond the scope of this communication. For such purposes, the already cited references have to be consulted.
It must be mentioned though, that numerous observations made with Amlaki, Triphala, and numerous other edible plant derived products have consistently revealed that their oral efficacies for stress responses modulating and other bioactivities increase with increasing numbers of treatment days (Kumar and Chatterjee, 2014a), and that this is most probably due to their modulating effects on microbial ecology inside the gastrointestinal tract (Thakur et al., 2014b). Although our current knowledge on the bactericidal constituents of Triphala and its constituents is far from being satisfactory, evidence now available on their diverse types of formulations strongly suggest that Gallic and Ellagic acids and their conjugates and soluble and insoluble polymers (commonly referred to as tannins) are their major secondary metabolites involved in bactericidal activities of their commercialized extracts (Biradar et al., 2008). It is now well recognized that gut microbial ecology plays a crucial role in the etiology, pathogenesis and progression of diabesity (Burcelin et al., 2011; Everard and Cani, 2013), and that such and other plant phenolics and their metabolites are regulators of gut microbial ecology (Bolca et al., 2013; van Duynhoven et al., 2011). Therefore, it is now apparent that proper understanding of their modulating effects on gut microbial ecology is an essential prerequisite for their more rational uses as dietary therapies against diabesity and associated mental health problems (Dinan and Cryan, 2012; Wang and Tang, 2015).
Curcuma longa (Turmeric)
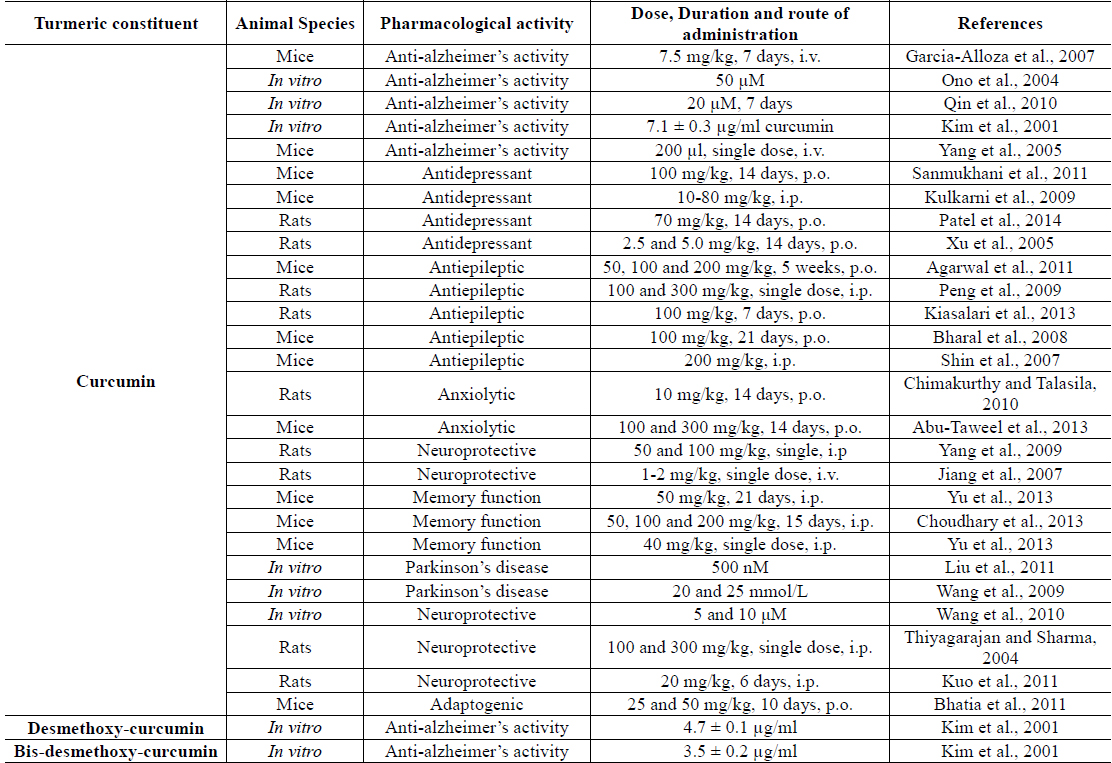
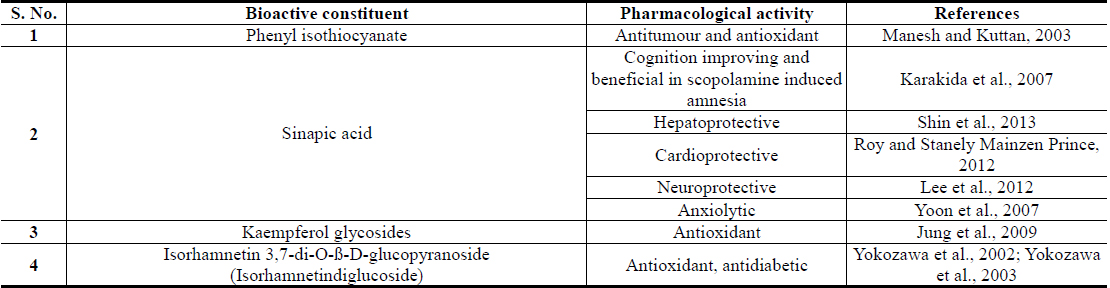
Curcuma longa (synonym: Curcuma domestica and commonly called turmeric) is one of the several plants of the Zingiberaceae family (genus: curcuma), well known since antiquity to the scholars and practitioners of traditionally known systems of medicine for diverse health benefits of their rhizomes. It is now widely cultivated and diversely processed in India, China, and many other countries for obtaining dried roots and other products from them for culinary as well as medicinal purposes (Li et al., 2011). In Ayurvedic and other traditionally known Indian system of medicine, turmeric is now often used as a general tonic and blood purifier and also for prevention and cure of inflammatory diseases. Currently, it is phytochemicallly and pharmacologically one of the more extensively studied edible plant derived products of medicinal interest. Numerous reviews and monographs describing diverse therapeutic possibilities offered by the plant and its bioactive secondary metabolites have appeared during recent years (Chaudhary et al., 2010; Gupta et al., 2013; Chempakam and Parthasarathy, 2008). Available preclinical and clinical information on anti-diabetic potentials of the plant has also been summarized in a recent issue of this journal (Ponnusamy et al., 2012). Most such reviews and reports often neglect though, that modulating effects of curcumin and other turmeric curcuminoids on brain functions could as well be involved in their modes of actions and health benefits. Some of the reports suggesting such possibility for curcumin and two other Turmeric curcuminoids are summarized in Table 5.
Amongst numerous known bioactive and therapeutically interesting secondary metabolites of turmeric identified and quantified to date, the three structurally and functionally analogous ones with antioxidative properties, i.e. curcumin, desmethoxycurcumin, and bisdesmethoxycurcumine (often collectively referred to as curcuminoids), have attracted the most attention of modern herbal researchers and drug discoverers (Aggarwal et al., 2007; Ahmed and Gilani, 2014; Alappat and Awad, 2010; Bradford, 2013; Grynkiewicz and Slifirski, 2012; Jayaprakasha et al., 2006; Lopresti et al., 2014; Sahebkar, 2013). Hereupon, by far a vast majority of preclinical and clinical reports deal mainly on two major obesity associated medical conditions, i.e. diabetes and cancer (Zhang et al., 2013). These efforts have not only continued to add preclinical and clinical evidences suggesting their therapeutic potentials against these and diverse other malnutrition associated health problems, but also have leaded to better understanding of medicinal phytochemistry and molecular pharmacology of curcuminoids (Grynkiewicz and Slifirski, 2012; Priyadarsini, 2014; Brodniewicz and Grynkiewicz, 2012). Some reports suggesting therapeutic potentials of Curcuma longa extracts against diabesity and associated comorbidities are summarized in Tables 6.
However, curcumin and curcuminoids are not the only bioactive secondary metabolites of Curcuma longa potentially involved in diverse traditionally known, or recently identified therapeutic potentials of turmeric (Aggarwal et al., 2013; Bhanumathy et al., 2013; Kasabri et al., 2014; Lekshmi et al., 2012). Although the list of bioactive phytochemicals, vitamins, and other micronutrients commonly consumed with turmeric, and which could also be involved in traditionally known health benefits of the spice have consistently been enlarged during the past few decades (Wang et al., 2014; Mirmiran et al., 2014; Vaidya, 2014), many questions concerning oral bioavailability, and the doses and treatment regimen necessary for obtaining therapeutic benefits from curcuminoids, or for that matter for any of the till now known bioactive secondary metabolites of turmeric, cannot yet be answered with any certainty (Anand et al., 2007). Some observations made in vitro, suggest that oral bioavailability of curcuminoids from turmeric could as well be higher than those of pure curcuminoids (Maheshwari, 2010). However, therapeutic relevance of these observations still remains questionable. This is mainly because numerous metabolic and behavioral effects observed after daily oral doses of curcuminoids seldom correlate with their blood levels observed after their single or repeated daily oral doses. Such is specially the case for their stress triggered brain function modulating effects in experimental animals observed after their oral doses commonly used for assessing their therapeutic potentials against diabesity and other metabolic disorders (Xia et al., 2011; Xia et al., 2006).
Taken together, these observations clearly suggest that health benefits of curcuminoids and other turmeric phytochemicals are either due to their bioactive metabolites, or that their observed broad spectrum of bioactivities are due to their non-systemic effects. Since turmeric is always consumed with other food ingredients containing diverse other bioactive molecules with broad spectrums of bioactivity profiles, it is almost certain that traditionally known health benefits of turmeric, cannot entirely be due to its curcuminoids contents, or on their blood levels observed after turmeric intake, only. Since analogous is the situation for almost all medicinally used edible plants and phytochemicals, attempts are now being made in several laboratories to use system-biology based pharmacological approaches for more rationally solving such problems (Goodacre, 2007; Nyanginja and Mponda, 2014; van Ommen and Stierum, 2002). However, during most such efforts attempts are made to better define the cellular and molecular mechanisms potentially involved in their modes of action, and as yet only very little attention has been paid to potential role of the so called microbiota-gut-brain axis in their observed effects in experimental animals and human volunteers and patients (Thakur et al., 2014b; Chiou et al., 2014; Greiner et al., 2014). However, the possibility that modulation of colonic microbiota could be involved in colon cancer prevention has recently been pointed out also (McFadden et al., 2014).
Some major difficulties encountered during efforts to extrapolate preclinical findings made with turmeric extracts and their known bioactivities in terms of traditionally known medicinal uses of the turmeric arise also from the fact that depending on harvesting and processing procedures used for medicinal value and the contents of curcuminoids and other bioactive constituents vary considerably (Pal et al., 2008). A recently reported computer assisted study have revealed that at least 200 structurally and functionally diverse bioactive phytochemicals can be expected to be present in a given turmeric sample (Balaji and Chempakam, 2010), and that many of them have them have adverse effect potential as well. Results of this in silico study have revealed that out of the 200 compounds screened, 184 were predictably toxigenic, 136 mutagenic, 153 carcinogenic, and 64 hepatotoxic, and that only 16 of them are devoid of any of these adverse effects detectable by the in silico procedure used. Although this report supplies an exhaustive list of bioactive secondary metabolites of Curcuma longa, predictive validity of the observations reported there must be judged with caution (Balaji and Chempakam, 2010). Numerous toxicological and safety reports on diverse types of products derived from the plant have always pointed out that their adverse effect can be expected only after their extremely high daily oral doses that cannot be consumed with every day meals or with Ayurvedic formulations containing them (Chainani-Wu, 2003; Joshi et al., 2003; Lao et al., 2006; Madhu et al., 2013; Micucci et al., 2013; Qureshi et al., 1992; Ulbricht et al., 2011; Velusami et al., 2013; Liju et al., 2013; Hasan et al., 2014).
Some high dose adverse effect potentials of phytochemicals often cited deal with their possible effect on liver functions (Kandarkar et al., 1998; Babu and Srinivasan, 1997), and those of gastrointestinal tract and skin (Fetrow and Avila, 1999). Unfortunately, as yet only a very few scattered reports on dose response studies necessary for predicting therapeutically interesting and safety margin of Curcuma longa extracts (other than those highly concentrated in curcumin and curcuminoids) have appeared (Micucci et al., 2013; Joshi et al., 2011). Results of one such recently reported preliminary study conducted in mice have revealed not only metformin (currently the drug of first choice for prevention and cure of diabesity) like stress response suppressing effects of fairly low daily oral doses of curcumin and some Curcuma longa extracts enriched in curcuminoids, but also suggest that even more than their 50 fold higher daily oral doses are fairly well tolerated by both male and female animals (Verma et al., 2015, Verma et al., 2014). Recent observations made in our laboratories with turmeric oil devoid of curcumin indicate that analogous is also the case for such oils (manuscript in preparation). These observations, and numerous other revealing that curcumin and diverse other turmeric constituents modulate the functions of stress responses mediated by shock proteins (Ali and Rattan, 2006; Speciale et al., 2011), add experimental evidences in support of the convictions that stress response regulating effects of turmeric derived products are involved in their modes of action, and that curcuminoids are not their only antidiabetic or stress response modulating bioactive constituents.
Phytochemicals, stress and diabesity
The term “diabesity” was initially coined during early 1970s to describe strong pathogenic links between obesity and type-2 diabetes (Sims et al., 1973). Although since then it has been well established that complex interactions between obesity, insulin resistance, and pancreatic ß-cell dysfunction cause type-2 diabetes, biological mechanisms and processes regulating the interplay among these impairments still remain to be better defined (Tschop and DiMarchi, 2012; Martinez and Milagro, 2015). However, it is now well recognized that even modest reduction of body weight can lead to significant improvements in glucose homeostasis of patients suffering from, or at risk to, diabesity (Martinez et al., 2014; Pati et al., 2014), and that uncontrollable stressful events and chronic stress states have significant and positive association with weight gains and type-2 diabesity (Adam and Epel, 2007; Dallman et al., 2005; Steptoe et al., 2014). This is most probably due to behavioral alterations triggered by environmental or mental stress, which eventually leads to addiction-like eating behavior (Ahmed et al., 2013; Ginty, 2013; Ginty et al., 2012; Scott and Johnstone, 2012; Sinha and Jastreboff, 2013). Although several questions concerning individual food components involved in “addictive eating behavior” still remain open (Ahmed et al., 2013; Davis, 2014; Meule et al., 2014), it is now almost certain that proper modulation of this behavior could indeed be an effective strategy for prevention of obesity associated physical and mental health problems (Meule et al., 2014; Murray et al., 2015; Pedram and Sun, 2015).
Amongst structurally diverse secondary plant metabolites with stress response modulating and other therapeutically interesting bioactivities, polyphenolics have attracted the most attention of modern nutritional researchers and pharmacologists (Lee et al., 2014; Nowak, 2015; Pa and Gazzaley, 2014). Although their health benefits are often considered to be due to their antioxidative properties, like other diverse phytochemicals (edible or not), they also possess broad spectrums of bactericidal, anti-inflammatory, immune function modulating, and numerous other therapeutically interesting bioactivities (Kennedy, 2014a; Kennedy, 2014b). Mounting preclinical and clinical evidences accumulated during the past few decades strongly suggest that their modulating effects on gut microbial ecology and digestive functions are also involved in their modes of actions (Thakur et al., 2014b). It is now well recognized that metabolic and psychological stress responses also alter gut microbial ecology, which in turn alters the functions of the gut-brain axis (Aroniadis and Brandt, 2013; Mayer, 2011; Moloney et al., 2014). According to the postmodern eco-physiological and pharmacological concept arising from these findings (Abedon, 2014), circulating blood levels of edible phytochemicals observed after their oral intake must not necessarily be very reliable indicators of their brain function modulating effects. Available information on metabolic fate of numerous edible polyphenolics (van Duynhoven et al., 2011) is also in agreement with this inference.
Eco-physiological and other studies have now established that secondary plant metabolites afford survival benefits to plants against enviorenmental stress (Kennedy, 2014a; Kennedy, 2014b; Trowbridge, 2014), and that structurally and functionally diverse edible phytochemicals possess pleiotropic protective effects against stress responses and are potentially useful for prevention and cure of diabetes, Alzheimer’s disease and other chronic silently progressing chronic diseases (Dembinska-Kiec et al., 2008; Leiherer et al., 2013; Davinelli et al., 2012; Vaiserman, 2014; Carriba and Comella, 2014; Franco and Cedazo-Minguez, 2014; Ruden and Lu, 2011). However, it has been also reported that depending on the components of whole-food some of them could as well worsen cognitive dysfunctions (Parrott et al., 2015). Available information on dose response relationship of numerous nutritive and other phytochemicals have revealed indeed that their protective or amplifying or adverse effects on stress responses depend on their daily doses and treatment regimen used, and that hereupon their beneficial effects often predominates (Calabrese et al., 2012; Le Bourg and Rattan, 2014; McClure et al., 2014). Such dose response relationships of bioactive molecules are due to their dose dependant modulating effects on diverse cellular adaptive stress responses (Birringer, 2011; Costantini et al., 2010) which often leads to their experimentally observed inverted U or J shaped dose response curves, or hormetic effects, in bioassays (Bao et al., 2014; Lushchak, 2014a). Evaluation of dynamics of such effects of edible phytochemicals are essential prerequisite for better understanding of their pharmacokinetic and pharmacodynamic properties necessary for obtaining health benefits offered by them (Lushchak, 2014b). This is because exposures to short term stress (hormetic stress) can strengthen subsequent response to stress, and prolonged stress exposures leads to toxic stress which shorten life span, and also leads to mental health problems (Lee et al., 2014; Epel and Lithgow, 2014; Scapagnini et al., 2014)