The growing demand for herbal remedial products has led researchers to exploit hitherto lesser known plant species as a source of several active compounds with special therapeutic properties. These include polyphenols, flavonoids, tannis, resins, alkaloids and saponins with curative effect (Salar and Seasotiya, 2011). Globally, 80% of the population relies upon drugs originated from plant world (World Health Organization, 2008). Use of herbal drugs is an important component of the traditional system of Indian medicine. Ethnobotanical surveys reveal that xerophytic plants possess an array of active compounds which can be exploited for a wide range of health benefits (Khare, 2007).
Owing to the intense demand of natural antioxidants to replace synthetic antioxidants in foods, a considerable interest have generated in the scientific community dedicated to research in the area of free radicals and antioxidants (Salar et al., 2011, 2012). Free radicals are molecules or molecular fragments containing one or more unpaired electrons in atomic or molecular orbitals (Halliwell and Gutteridge, 1999). Reactive oxygen species (ROS) as well as reactive nitrogen species (RNS) are produced during normal cellular metabolism in living organisms. Overproduction of these reactive species cause potential biological damage termed as oxidative stress and nitrosative stress, respectively (Ridnour et al., 2005; Salar et al., 2013).
The excess ROS/RNS can damage cellular lipids, proteins or DNA inhibiting their normal function (Valko et al., 2006). Because of this, oxidative stress has been implicated in a number of human diseases including cancer, malaria, cardiovascular disease, diabetes and many other ailments related to ageing (Honda et al., 2004). Living beings have developed a series of defense mechanisms against the deleterious effects of free radicals including (i) preventive mechanism, (ii) repair mechanism, (iii) physiological defenses, and (iv) antioxidant defenses. Antioxidant defenses include enzymatic (superoxide dismutase, glutathione peroxidase, catalase) and non-enzymatic antioxidants. The later are represented by ascorbic acid (Vit. C), - tocopherol (Vit E), glutathione (GSH), carotenoids, flavonoids etc. (Valko et al., 2007) which are present in plenty in the plant world.
Hence the aim of this study was to carry out the antioxidant and free radical scavenging activity of sequential extracts of succulent stem of
>
Plant material and extraction
The stem of
Ten grams of dried powder was suspended in 100 ml of respective solvent (water [AqE], acetone [AE] and methanol [ME]) and kept on a rotary shaker for 24 h. The extract was filtered through eight layers of muslin cloth and centrifuged at 5000 g for 15 min. Supernatants were collected and the extracts were concentrated to make the final volume one-fourth of the original volume using rotary vacuum evaporator under reduced pressure at 40℃.
Folin-ciocalteu reagent and organic solvents used for extraction were of analytical grade and purchased from Fischer Scientific. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2-thibarbituric acid (2-TBA), deoxyribose, ferrozine, potassium ferricyanide, ferric chloride, EDTA, hydrogen peroxide, L-ascorbic acid, sodium hydroxide, trichloroacetic acid, sodium carbonate, phoaphate buffer, butylated hydroxyanisole (BHA), ferrous chloride and butylated hydroxytoluene (BHT) were from Hi-media Pvt. Ltd., Mumbai.
>
Total phenolic content (TPC) determination
Total phenolic contents (TPC) of the extracts were determined using Folin-Ciocalteu (FC) reagent following Yu et al. (2002) with slight modifications. Briefly, 100 μl of extracts were mixed with 0.5 ml of FC reagent in a 10 ml volumetric flask. Then 1.5 ml aqueous solution (20%, w/v) of sodium carbonate anhydrous was added, vertexed and incubated for 15 min at room temperature. The flask was filled with distilled water to volume. The absorbance was read at 765 nm, subtracting the value of a control solution consisting of distilled water instead of extracts. The amount of TPC was calculated as gallic acid equivalents (GAE) from the standard calibration curve of gallic acid and expressed as mg gallic acid equivalents per gram of sample.
The extracts were measured for hydrogen donating or radical scavenging ability using the stable DPPH radical according to the modified method of Yen and Chen (1995). The reaction mixture was prepared containing 300 μl of extract of varying concentration (50 - 300 μl/ml) and 2 ml of 100 μM DPPH (4 mg DPPH in 100 ml methanol). The absorbance of mixture was measured at 517 nm against the blank (containing solvent instead of extract) in a spectrophotometer after 10 min of incubation at room temperature. L-ascorbic acid was used as control. The percent DPPH inhibition was calculated by the equation:
% inhibition= A0 - A1 / A0 × 100
Where A0 was the absorbance of control and A1 was the absorbance of reaction mixture.
>
Deoxyribose degradation assay (site specific)
The site-specific deoxyribose assay was performed following Arouma et al. (1987) and Halliwell et al. (1987) with certain modifications. Briefly, the extracts (from 1 - 100 μg/ml) were mixed with a Haber-Weiss reaction buffer [10mM FeCl3, 1mM phosphate buffer (pH 7.4), 10 mM H2O2, 10 mM deoxyribose, and 1mM L-ascorbic acid] and the final volume of all mixtures was made to 1.0 ml. The mixture was then incubated at 37℃ for 1h and heated at 80℃ for 30 min. with 1 ml of 2-TBA (0.5% 2-TBA in 0.025 M NaOH, 0.02% BHA) and 1ml of 10% trichloroacetic acid (TCA) in water bath for 45 min. After cooling, absorbance of the mixture was measured at 532 nm in a spectrophotometer. The percentage inhibition was calculated employing the formula as given for DPPH scavenging assay and was correlated with total phenolic content.
Reducing power of the extracts was determined following the method of Oyaizu (1986). Briefly, to varying concentrations of extract (1 ml) were added 2.5 ml phosphate buffer (0.2M, pH 6.6) and 2.5 ml potassium ferricyanide (1%). The reaction mixture was incubated at 50℃ for 20 min. Aliquots (2.5 ml) of trichloroacetic acid (10%) were added to the mixture and centrifuged at 9500 rpm for 10 min. The upper layer of solution (2.5 ml) was recovered and mixed with distilled water (2.5 ml) and 2.5 ml FeCl3 (0.1%) and the absorbance was recorded at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicated increasing reducing power of the extracts.
>
Chelating effects on ferrous ions
The chelating effect on ferrous ions was determined according to the method of Dinis et al. (1994). The extracts (0.25 ml) were mixed with 1.75 ml of methanol and 0.25 ml of 250 mM FeCl2. This was followed by the addition of 0.25 ml of 2 mM ferrozine, which was left to react at room temperature for 10 min. before determining the absorbance of the mixture at 562 nm. The chelating effect (%) was calculated from the formula as given for DPPH scavenging assay.
The mean values and the standard deviations were calculated from the data obtained from three independent experiments. Statistical differences at
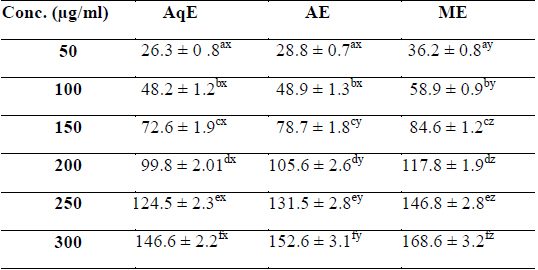
Phenolic compounds are aromatic secondary metabolites that have been extensively investigated in medicinal plants, fruits and vegetables (Djeridane et al., 2006). Phenolics are associated with colour, sensory qualities, nutritional and antioxidant properties (Robbins, 2003). The total phenolic contents of different extracts of
[Table 1.] Total phenolic content (mg/g GAE) of various extracts of succulent stem of E. trigona
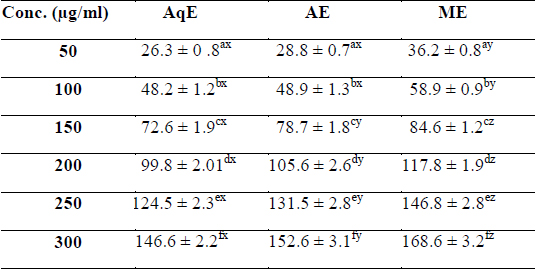
Total phenolic content (mg/g GAE) of various extracts of succulent stem of E. trigona
>
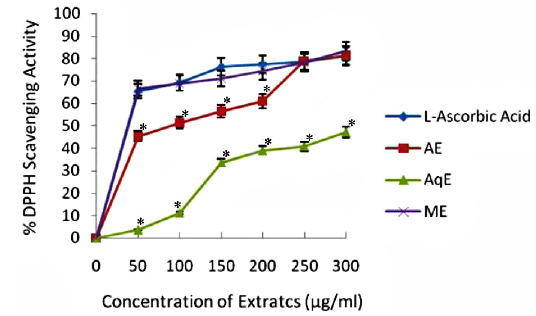
DPPH Radical Scavenging Activity
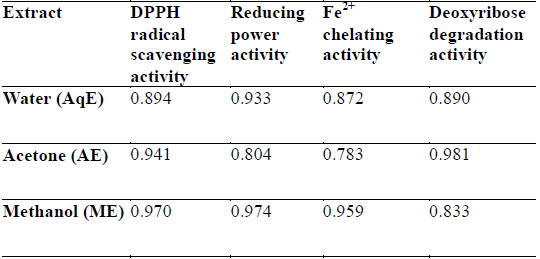
DPPH is a stable free radical and is widely used to assess the antioxidant activity of natural extracts or pure compounds to act as free radical scavenger or hydrogen donors. In the present study, all the extracts depicted significantly higher tendency to scavenge the DPPH radicals in a dose dependent manner and steadily increased with increase in extract concentrations (Fig. 1). ME extracts showed strongest antiradical activity (83.25% at 300 μg/ml) which was even higher than the standard (L - ascorbic acid 81.30% at 300 μg/ml). Among the other extracts, AE also showed notably higher quenching ability of the DPPH radical, however AqE were less effective scavenger of the DPPH radical (Fig. 1). A good correlation (R2) between DPPH scavenging activity and TPC was obtained (Table 2) which indicates that phenolic compounds are directly responsible for antioxidant activity.
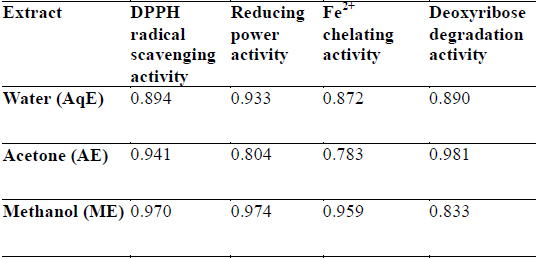
Correlation coefficients (R2) for phenolic compounds and antioxidant activity relationship of different extracts of E. trigona.
>
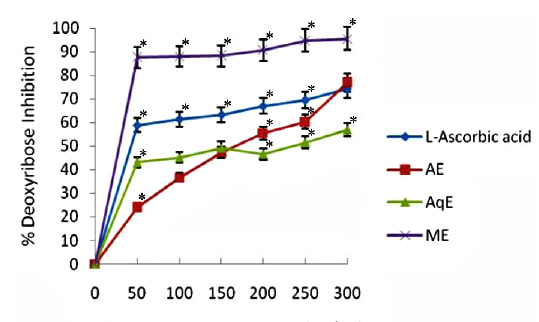
Deoxyribose degradation activity
The results obtained in deoxyribose degradation assay to prevent 2-deoxy-D-ribose oxidation mediated by OHㆍ radicals are shown in Fig. 2. The reduction ability of all tested extracts increased dependently with increasing concentrations in the site-specific assay. In general, it was observed that all the extracts displayed a protective effect depending on the concentration of the extract. Methanol extracts of E. trigona showed significantly higher (
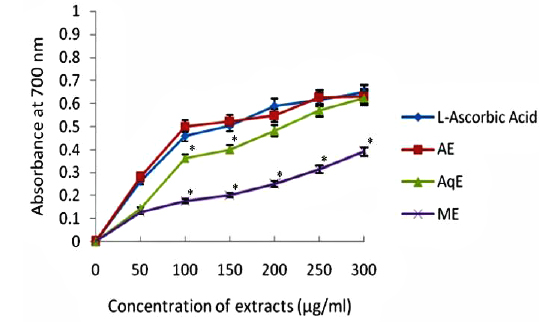
Fig. 3 depicts the results of relative reducing power of all the extracts of E. trigona and L - ascorbic acid (standard antioxidant compound). It is clear from the results that ability of AE and AqE was comparable with standard (L – ascorbic acid) in reducing Fe3+ to Fe2+ and there was no significant difference of activity. The activities of water extracts (AqE) were inferior (0.391 at 300 μg/ml) to L - ascorbic acid (0.647 at 300 μg/ml) and were significantly (
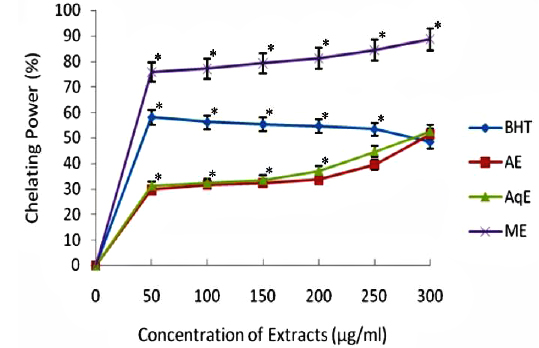
The ferrous state of iron can stimulate lipid peroxidation by the Fenton reaction and is the most powerful pro-oxidant among various species of metal ions. The results of chelating ability of various extracts of
Free radical scavenging activity of putative antioxidants may possibly result by the synergy between a number of mechanisms. These may include prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxidises, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging (Salar and Seasotiya, 2011; Shahat et al., 2014). Owing to complex nature of the phytochemicals present (Hall and Cuppett, 1997), the antioxidant activity of plant extracts cannot be evaluated by a single method. In order to explore these additional mechanisms, several antioxidant assays, namely DPPH, reducing power, deoxyribose degradation and chelating power assay were employed in the present investigation to evaluate antioxidant activity of
It is reported that the antioxidant activity of plants is attributed to the presence of phenolic compounds (Chizzola et al., 2008; Conde-Hernandez and Guerrero-Beltran, 2014; Salar and Dhall, 2010) which are generally very active in scavenging DPPH free radicals due to their fast electron transfer process, while hydrogen atom abstraction becomes a marginal reaction path (Foti et al., 2004). In the present study, four assays were employed to assess the antioxidant activity of
Hydroxyl radical (OHㆍ) is the most reactive ROS and attacks almost every molecule in the body resulting in peroxidation of cell membrane lipids and in formation of malondialdehyde, which is mutagenic and carcinogenic (Salar and Seasotiya, 2011). The results of site specific deoxyribose degradation assay in the present study have suggested that AE and AqE of
Reducing property of extracts shows that antioxidant compounds are electron donors able to reduce the oxidized intermediates of lipid peroxidation process (Nagmoti et al., 2012). In the present investigation it is clear that the reducing power was a function of concentration and increased with increasing concentration of the tested extracts (Fig. 3), however, all extracts had less reducing power than ascorbic acid (standard) which showed a reducing power of 0.647 at 300 μg/ml. Further, there was a good correlation between phenolic compounds and reducing power of all extracts (Table 2) suggesting that polyphenols may be the principal constituents responsible for such properties.
Transition metals viz., Fe2+ and Cu2+, can catalyze the generation of reactive oxygen species such as hydroxyl radical (OHㆍ) and superoxide anion (O2 −), (Stohs and Bagachi, 1995). In particular, Fe2+ generates ·OH by the Fenton reaction, which accelerate the lipid peroxidation chain reaction. In addition, Fe2+ catalyses the breakdown of lipid peroxides, that leads to the formation of volatile oxidation products responsible for offflavour development (Surendraraj et al., 2013). At the highest concentration tested (300 μg/ml), all extract exhibited Fe2+ chelating activity and were higher than the standard BHT. ME, however, showed highest Fe2+ chelating activity as compared to other extracts in a concentration dependent manner (Fig. 4). The higher Fe2+ chelating activity of ME in all concentrations tested may be due to the presence of specific phenolic compounds, which have been reported to be good chelating agents. Moreover, there was a good correlation (R2 0.959) between chelating power of phenolic compounds and methanolic extracts (Table 2).
The results of this study demonstrated that the green succulent stem of