Because of avaricious global energy consumption, and serious environmental issues, the demand for clean energy technology has grown to a significant level, and the development of emerging effective energy devices and resources has become one of the most important topics in recent decades, and has attracted passionate research [1]. Among encouraging alternative sources of energy, supercapacitors are one of the promising electrochemical energy storage devices, with high power characteristics compared to batteries, high energy density compared to conventional capacitors, and long cycling times [2-6].
Hence, supercapacitors have been practically used in various applications, and offer potential benefits in communications, consumer electronics, aviation, transportation and associated technologies [7-9]. It is well known that the performance of electrochemical double layer capacitors (EDLCs) typically depends on the high specific surface area and highly reversible redox reactions of the electrode materials [10]. Therefore, various carbonaceous materials, such as carbon nanotubes, activated carbons, carbon blacks, carbon aerogels and carbon fibers have been investigated as electrode materials for supercapacitors. Comparatively, carbon nanofibers (CNFs) have gained great importance in recent years due to their large axial ratio [11,12]. Presently, CNFs are extensively applied in many fields such as fuel cells, EDLCs, hydrogen energy and lithium-ion cells [13-17].
On the other hand, various oxides of transition metals, such as manganese [18], cobalt [19], nickel [20], and tin [21] have been used as electrode materials for EDLCs, because of low cost and non-toxicity [22]. Furthermore, it has also been reported that Co based materials have good electrochemical activity [23] and the latest studies have shown that this metal does have good electrocatalytic properties if it can be supported by the proper co-catalyst [24].
CeO2 has received considerable attention due to its remarkable properties and wide applications in various fields such as oxygen permeation membrane systems [25,26], low-temperature water-gas shift reaction [27,28], oxygen sensors [29,30], glass-polishing materials [31,32], and fuel cells [33-36].
In this paper, we demonstrate a methodology for the synthesis of Co/CeO2-doped CNFs, which are introduced as an electrode of electrochemical capacitors (EC). The proposed nanostructures were synthesized by the carbonization of an electrospun mat composed of cerium acetate, cobalt acetate, and poly(vinyl alcohol) (PVA) in an argon atmosphere at 700℃.
Cobalt (II) acetate tetrahydrate (CoAc, 98%; Aldrich, St. Louis, MO, USA) and cerium (III) acetate hydrate (CeAc, 99.9%; Aldrich) were used as the precursors for Co and CeO2 while PVA with a molecular weight 65,000 g/mol (Aldrich) was used as the precursor for the carbon fiber.
A mixture containing 75 wt% CoAc and 25 wt% CeAc was mixed with 15 g PVA aqueous solution (10 wt%). Finally the mixture was stirred at room temperature for 5 h to obtain a transparent mixture. The obtained sol-gel was electrospun at a high voltage of 21 kV using a direct current (DC) power supply at room temperature. The produced mats were dried at room temperature for 10 h and then under vacuum overnight at 70℃ and finally carbonized at 700℃ for 6 h in inert atmosphere (The diagram of the general synthesis of the nanocomposite is depicted in Scheme 1).
0.002 g of the nanocatalyst, 20 μL of Nafion solution (5 wt%; Aldrich) and 400 μL of isopropanol were mixed together and sonicated for 45 min at room temperature. The dispersed nanocatalyst (15 μL) was spread by micro pipette onto the active surface of the glassy carbon electrode, which was then subjected to a drying process at 75℃ for 30 min.
The electrochemical performance of the produced electrode was characterized by cyclic voltammetry using a three electrode electrochemical cell (VersaSTAT 4, USA) at room temperature in a 1 M KOH solution. The three electrode cell consisted of a working electrode, and a Pt wire and an Ag/AgCl electrode were used as the auxiliary and reference electrodes, respectively.
Characterization experiments were carried out at room temperature. The phase of the CNF’s was identified by X-ray diffractometer (XRD, Rigaku, Tokyo, Japan) with Cu-Kα (λ = 1.54056 Å) radiation operating at 45 kV and 100 mA over a range of 2θ angle from 10° to 80°, scanning at a rate of 4°/min. The morphology, crystalline size, and crystal structure of the sample were determined by field emission scanning electron microscopy (FESEM; Hitachi S-7400, Hitachi, Tokyo, Japan) coupled with rapid energy dispersive analysis of X-ray (EDX), transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM; JEM-2200FS, JEOL, Tokyo, Japan) operating at 200 kV equipped with EDX (JEOL).
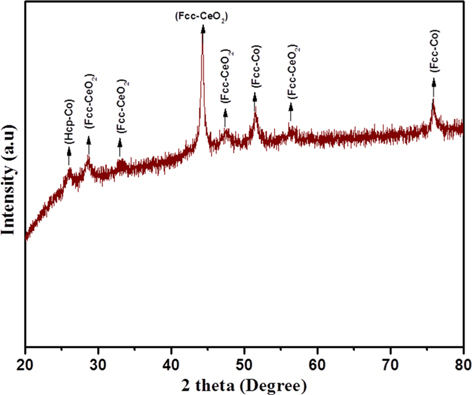
Fig. 1 shows the XRD pattern of the Co/CeO2-CNFs, which were carbonized at 700℃ for 6 h. As shown in the figure, strong diffraction peaks were observed at 2
Regarding cobalt acetate, the XRD results show that the cobalt acetate has been decomposed to metallic Co, as the strong diffraction peaks at 2
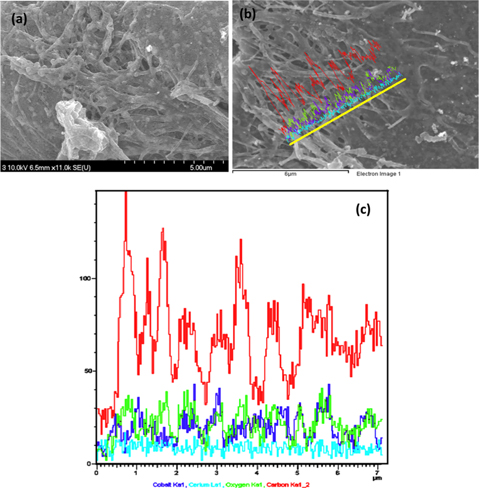
FESEM images of the electrospun nanofibers containing CoAc and CeAc precursors after carbonization in an inert environment revealed good morphology. The nanofibers (NFs) are shown in Fig. 2a. As shown in the figures, nanofibers were formed with complete, clear and regular shapes with smooth surfaces, due to the small size of the bimetals. Furthermore all the bimetallic particles are on the surface of the nanofibers.
To properly understand the composition and elementary analysis of the obtained Co/CeO2-CNFs, FESEM-EDX was carried out. The results are presented in Fig. 2b and c.
Fig. 2c presents the EDX result corresponding to the line shown in Fig. 2b. As shown in Fig. 2c, Co, Ce, and O have the same elemental distribution along with the chosen line; moreover, the carbon elemental distribution confirms complete covering of the Co and CeO2 nanoparticles, as carbon is present at all points along the chosen line.
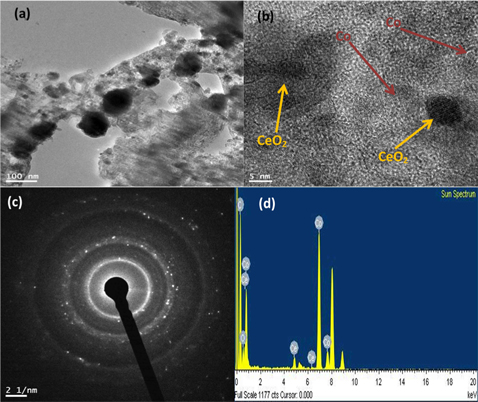
Analysis of the internal structure of the as-prepared bimetallic CNFs was done using transmission electron microscope (TEM), and high resolution transmission electron microscope (HR-TEM). Fig. 3 shows the obtained results; Fig. 3a and b displays low and high resolutions, respectively.
The bimetallic CNFs morphology is perfect in the image and the small black crystalline nanoparticles distributed on the nanofibers are surely Co and CeO2 nanoparticles. The Co and CeO2 nanoparticles are indicated in the HR-TEM image by dark yellow and dark red arrows in Fig. 3b.
In order to confirm the existence of C, O, Ce, and Co in the nanofibers, TEM-EDX analysis was carried out. The results are presented in Fig. 3d. It was observed that the sample contains C, O, Ce, and Co; no other elemental impurities are detected.
Fig. 3c shows the distinctive rings of the selective area electron diffraction pattern (SAED); the SAED image indicates the good crystallinity of the synthesized nanostructure.
The prepared Co/CeO2 CNF’s were then used as electrode materials for a supercapacitor and its performance was analyzed using cyclic voltammetry.
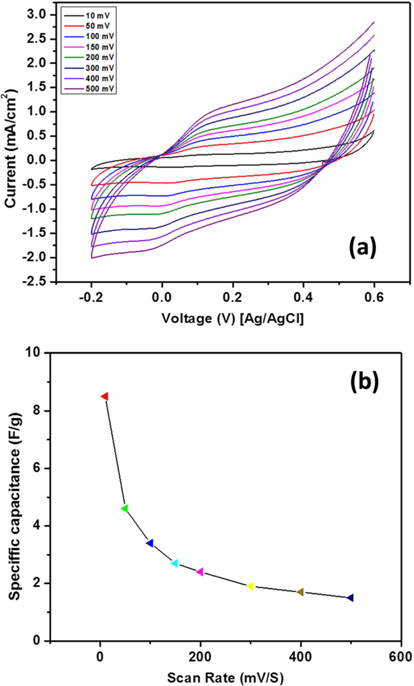
The cyclic voltammetry (CV) curves of the nanocatalyst studied in a 1 M KOH solution at various scan rates are shown in Fig. 4a, and all the curves are perfectly rectangular in shape without any redox peaks, which shows that the sample has good capacitance behavior.
It is well known the capacitance value depends upon scan rate so the effect of various scan rates on capacitive behavior was studied, and results are presented in Fig. 4b.
The specific capacitance of the samples were calculated using the following eq [37,38]:
Here,
where N is the number of points in the (CV cycle). As shown in Fig. 4, the corresponding specific capacitance of the introduced nanocatalyst is high at low scan rate (8.5 F/g) because the diffused ions from the solution can more easily access the electrode surface, which leads to more surface adsorption/desorption of ions [39], Moreover, the specific capacitance is relatively stable at the high scan rates.
CNFs doped with Co/CeO2 nanoparticles with good morphology were prepared by the electrospinning of a sol-gel composed of cerium (III) acetate hydrate, cobalt (II) acetate tetrahydrate and poly(vinyl alcohol). The synthesized nanofibers can be utilized as operative inexpensive electrodes in electrochemical supercapacitors.