Limited conventional energy resources and serious environmental calamities have motivated researchers to find new and efficient sources of energy. The considerable efforts devoted to this end include the development of bio-diesel, solar cells, coal liquefaction/gasification technologies, and fuel cells. Hydrogen is considered by many an ideal energy source owing to its renewable and clean energy characteristics. In addition, it mostly produces water, which is eco-friendly compared to the byproducts of many other energy sources [1-6]. To use hydrogen as an energy source, various hydrogen storage methods such as the use of metal hydrides, liquefied hydrogen, and adsorption of hydrogen in porous materials have been studied [7-14]. The adsorption of hydrogen in porous materials is particularly suitable for hydrogen storage, which is critical for suitably utilizing hydrogen energy, owing to the reversibility and stability of this method. To date, the wide ranging attempts to develop hydrogen storage mechanisms include studies on carbon materials [15-17], metal-organic frameworks, and zeolites [18-21]. Carbon materials offer many advantages for hydrogen storage, such as easy preparation, suitable surface functional groups, low mass density, thermal stability, and hydrophobicity [22-24]. Carbon aerogels (CAs) in particular have been recognized as potential hydrogen storage materials because of their suitable structural properties, controllable mass densities, high specific surface areas, and mesopore volumes. However, it is necessary to modify the surfaces of carbon materials in order to reach the hydrogen storage capacities determined by the US Department of Energy (DOE).
Carbon materials containing transition metals such as platinum, copper, vanadium, and nickel exhibit attractive hydrogen storage properties [10,22,25]. Nickel is particularly promising since it is easily obtained on Earth, and it is inexpensive compared to other metals and enhances hydrogen storage properties. Improved hydrogen storage capacities have been obtained with nickel-plated porous carbon nanofibers owing to the spillover of hydrogen molecules onto the metal-carbon interfaces. In general, the presence of nickel particles on carbon materials has been shown to enhance the hydrogen storage capacity [3,9,19].
In this work, CAs were chemically activated by KOH to produce well-developed pore structures and ultimately increase the hydrogen storage capacities. Nickel-loaded activated carbon aerogels (ACAs/Ni hybrid materials) with various nickel content were prepared. Ni particles were loaded onto ACAs to introduce hydrogen-favorable active sites onto the ACA surfaces and improve the hydrogen storage capacities.
The CAs were prepared using resorcinol as the carbon precursor and formaldehyde as the initiator. Resorcinol (7.5 g) was dissolved in a solution of distilled water (75 mL) and formaldehyde (25 g). Sodium carbonate was then added to this solution, and the mixture was stirred for 3 h in an open container and dried for 3 h at room temperature. Subsequently, the wet gel was dried at 80℃ for 48 h and then immersed in an acetone solution for 24 h without stirring. The aerogels were obtained after drying at 80℃ for 24 h. Prior to use, the aerogels were carbonized in a furnace with a heating rate of 2℃ min−1 under a N2 gas atmosphere. The CAs were treated with KOH solution at 60℃ overnight, with a KOH/CA mass ratio of 2:1. The prepared KOH/CA mixtures were then heated to 900℃in a furnace under a N2 gas flow (heating rate 2℃ min−1, flow rate 200 cm3/min). The resulting products were then washed with distilled water until a neutral pH was attained. The samples were dried in an oven at 80℃for 24 h to obtain the ACAs.
To synthesize the ACA/Ni hybrid materials, the ACA was added to 100 mL of ethylene glycol and ultrasonicated for 1 h. Nickel sulfate hexahydrate (0.2, 0.5, 1, and 2 wt% of NiSO4·6H2O per 1 g of ACA) was dissolved in ethylene glycol and the solution was mechanically stirred for 4 h. NaOH was added to adjust the pH of the solution to neutral values. To remove oxygen and the organic byproducts, formaldehyde was added and stirred at 90℃ for 2 h under an Ar atmosphere. Finally, the ACA/Ni hybrid materials were washed with de-ionized water and dried in a vacuum oven for 24 h [3].
X-ray photoelectron spectroscopy (XPS) measurements were conducted with a monochromated Al Kα X-ray source. Survey scans for Ni2p were shown in the 854-859 eV binding energy range, with a pass energy of 0.1 eV. A non-linear least squares curve-fitting program (Peak-Fit version 4) with a Gaussian-Lorentzian mix function and Shirley background subtraction was employed to interpret the XPS peaks. To examine the porous textural characteristics of the samples, N2/77 K adsorption-desorption isotherms were obtained using a Belsorp Max instrument (BEL Japan, Inc., Toyonaka, Japan). The samples were degassed at 473 K for 6 h to reduce the residual pressure below 10−6 mmHg. The specific surface areas were calculated using the Brunauer-Emmett-Teller (BET) equation. The micropore size distributions were obtained by applying the Horvath-Kawazoe (H-K) method.
The hydrogen storage measurements were conducted at 298 K/100 bar, conditions that are compatible with future electric-vehicle applications. Prior to the measurements, the samples in the chamber were degassed at 473 K for 12 h. After the chamber was cooled to room temperature, the samples were purged with flowing He and stored under a He atmosphere before further measurements. Hydrogen was supplied to the chamber until a pressure of 100 bar was achieved. Ultra-high-purity hydrogen gas (99.9999%) was used to exclude the influences of moisture and other impurities. Finally, the hydrogen storage capacity was determined by a volumetric measurement method.
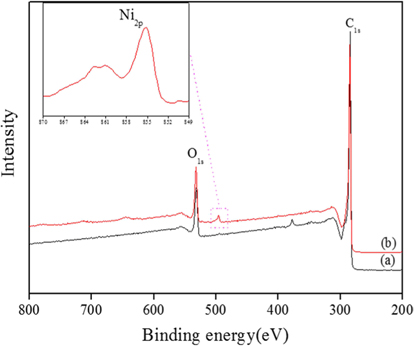
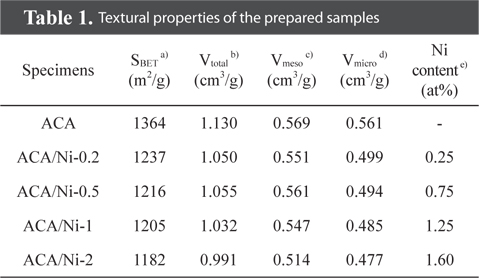
An XPS analysis was carried out to obtain chemical species information of the ACA/Ni hybrid material. As shown in Fig. 1, the spectra of the ACA showed C1s and O1s peaks, but no Ni2p peaks, whereas the spectra of the ACA/Ni hybrid materials exhibited C1s, O1s, and Ni2p peaks. The presence of oxygen in the spectra is attributed to natural auto-oxidation. As shown in Table 1, the amount of surface Ni atoms gradually increased as the ACA/Ni ratio increased from 0.2 to 2.0. The intensities of the Ni2p peaks in the survey spectra of the ACA/Ni hybrid materials were clearly higher than that of ACA, thus demonstrating that the ACA and nickel particles are hybridized.
[Table 1.] Textural properties of the prepared samples
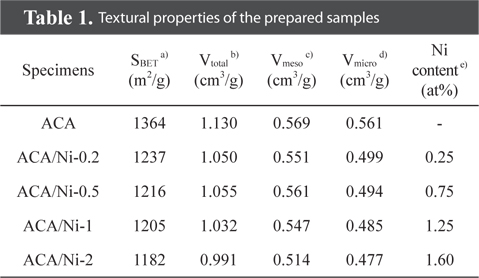
Textural properties of the prepared samples
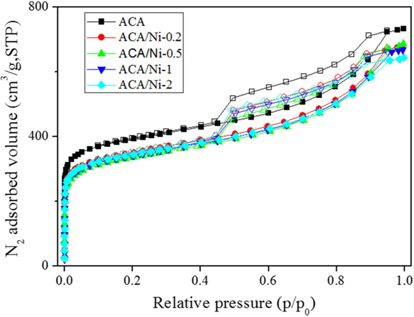
The N2/77 K adsorption-desorption isotherms of the prepared samples with various nickel content are shown in Fig. 2. A typical type-IV isotherm with a hysteresis loop at relative pressure above 0.4 is observed, reflecting the influence of capillary condensation and the inter-tubular structure of the prepared samples [26,27]. While this type of isotherm indicates mesoporous materials, isotherms at a relative pressure below 0.1 are attributed to micropores, suggesting that the prepared samples are both microporous and mesoporous. The shapes of the isotherms of the ACA/Ni hybrid materials were consistent with the isotherm of the ACA. However, slight decreases in the specific area and total pore volume were observed owing to the blocking and filling of micropores and mesopores with nickel particles. The textural properties of the samples are shown in Table 1 to shed light on the pore structure. From these results, the adsorption volume, which depends on the specific surface area and total pore volume of the samples, gradually decreases as the nickel content increases, and this is ascribed to the nickel particles blocking the pores on the ACA surface.
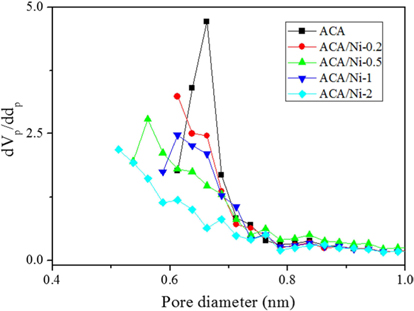
Fig. 3 shows the micropore size distributions of the prepared samples with various amounts of nickel, determined using the H-K method. The micropore sizes ranged from 0.4 to 1.0 nm; this range also contains the optimal pore size range (0.6-0.7 nm) for hydrogen adsorption [28-30]. The micropore size distributions of the ACA were uniform, in contrast with those of the ACA/Ni hybrid materials. The results also showed that the the highest volume peaks of the prepared samples decreased in height as the nickel content increased.
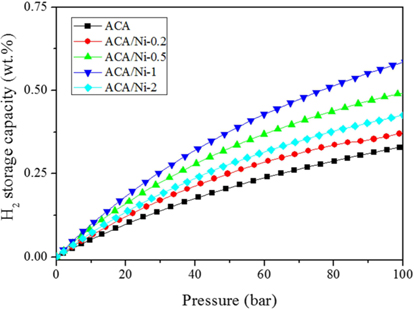
The hydrogen storage capacities of ACA and ACA/Ni hybrid materials containing various amounts of nickel measured at 298 K/100 bar are shown in Fig. 4. The results show that the hydrogen storage capacities of the ACA/Ni hybrid materials are higher than that of the untreated ACA. Furthermore, the highest hydrogen storage capacity of the ACA/Ni hybrid materials (1 wt% of nickel per 1 g of ACA, ACA/Ni-1) is almost two times that of the untreated ACA. Normally, the hydrogen storage capacities of carbon materials depend strongly on the pore volume, specific surface area, and the accessible micropores where hydrogen adsorption mainly occurs. However, the results indicated that the hydrogen storage capacities of the ACA/Ni hybrid materials increased, despite that the micropore volume and specific surface area decreased because of the Ni particles on ACA. The above results clearly show that Ni particles are hydrogen-favorable sites by a spillover effect and enhance the hydrogen storage capacities of the ACA/Ni hybrid materials. In addition, in the case of metal-deposited adsorbents, charge transfer occurs between the metal and the adsorbent. The positively charged metal atoms polarize the hydrogen molecules, and the hydrogen molecules bond with the metal atoms. It is critical that hydrogen is adsorbed on the mesopores as well as the micropores. Both factors play an important role in determining the hydrogen storage capacity in the presence of Ni particles. Even though the pore volume and specific surface area decreased as a result of the blocking or filling of micropores, the adsorption of hydrogen atoms by the spillover effect is not affected by any blocking effect, as revealed by the improved hydrogen storage capacities [31-33]. These results show that the hydrogen storage capacities of ACA/Ni hybrid materials deposited with Ni (0.2, 0.5, and 1 wt% per 1 g of activated carbon aerogel) gradually increase as the Ni content steadily rise owing to adsorption in the presence of Ni. Thus, ACA/Ni-0.2 and 0.5 with low Ni content showed lower adsorptions than ACA/Ni-1. It was shown that the hydrogen storage capacity of ACA/Ni-2 decreased because of filling and blocking by the large amount of Ni particles. In conclusion, ACA/Ni-1 has optimum Ni content on the ACA surface and the nickel was well dispersed. Furthermore, ACA/Ni-1 has optimum pore size for hydrogen storage (as previously mentioned in Fig. 3).
Consequently, the highest hydrogen storage capacity of the ACA/Ni hybrid materials was 0.6 wt%, which was two times higher than that of untreated ACA. Although this is still lower than the goal established by the DOE, it nevertheless suggests the potential of carbon materials for hydrogen storage and an important relationship between the carbon material structure and suitable metals.
In this work, ACA/Ni hybrid materials were prepared by KOH activation of CAs and nickel nanoparticle deposition on ACAs in order to increase their hydrogen storage capacity. The specific surface area and micropore volume of the ACA decreased with the introduction of nickel owing to the pore blocking and filling effect of the Ni particles on the ACA surface. The hydrogen storage capacity of the untreated ACA sample was approximately 0.3 wt%, whereas that of the ACA/Ni-1 hybrid sample was 0.6 wt%. The presence of Ni particles introduced hydrogen-favorable sites, which can lead to an improved hydrogen storage capacity due to the catalytic characteristics of nickel. Based on these results, we suggest that ACA/Ni hybrid materials can potentially be used as hydrogen storage adsorbents given that the raw materials are easily obtainable and inexpensive.