Over the years, the furnace has been used as a common heating method to manufacture activated carbon. In a furnace, heat is transferred through conduction and convection. The outer surface of the sample is in contact with the generated heat, which slowly diffuses inwards as a result of the thermal gradient between the surface and the core of the material’s particles. Another method of heating employs microwave irradiation. Even though it is less energy- and time-consuming, the microwave method has several critical issues with respect to temperature control and thermal runaway, especially in the scaling-up of the microwave heating process [1].
Generally, the activation of a carbonaceous precursor can be performed through physical (steam, air or CO2) or chemical activation (activators such as ZnCl2, KOH, etc.) or a combination of both. The chemical activation is normally preferable over physical activation since it is a faster process with a lower activation temperature. Moreover, the activated carbon produced via chemical activation usually possesses high specific surface area (as determined by the Brunauer-Emmett-Teller, BET method), good pore development and high carbon yield [2,3]. In recent years, potassium salts such as KOH and K2CO3 have been widely used in the manufacture of low cost activated carbon. It has been found that activated carbon prepared by KOH activation is highly microporous when compared to that produced through ZnCl2 or H3PO4 activation [4-6]. Besides, KOH also enhances the specific surface area and the formation of—OH functional groups on the carbon surface [7].
Over the past 5 years, many advantages of KOH activation have been revealed in the literature [8]. However, the adverse drawbacks of employing KOH have been overlooked in many of the published studies. In this paper, the preparation of activated carbon by KOH activation using conventional heating is reviewed and discussed. The limitations and implications of using KOH in the activation process are highlighted. The selection of appropriate potassium salts for activated carbon preparation is also recommended.
The physical preparation of activated carbon is comprised of two major processes, namely, carbonization and activation of the carbonized sample [4]. Chemical activation is a single step process, as both carbonization and activation occur simultaneously at temperatures ranging between 400℃ and 700℃, which is lower than that of physical activation [9]. However, in some cases, additional carbonization or a pre-carbonization step is performed to produce char prior to chemical impregnation and activation [5,4,10-13]. Thus, potassium hydroxide activation can be achieved through either direct chemical activation or char-impregnated chemical activation.
In direct chemical activation, a selected carbonaceous precursor is first dried overnight to remove moisture and then chemically treated at a desired impregnation ratio (weight of KOH over weight of precursor). The impregnated solid is then heated in a furnace at a specified temperature and time. Carbonization of the precursor is often omitted when the impregnated solid is already suitable for activation.
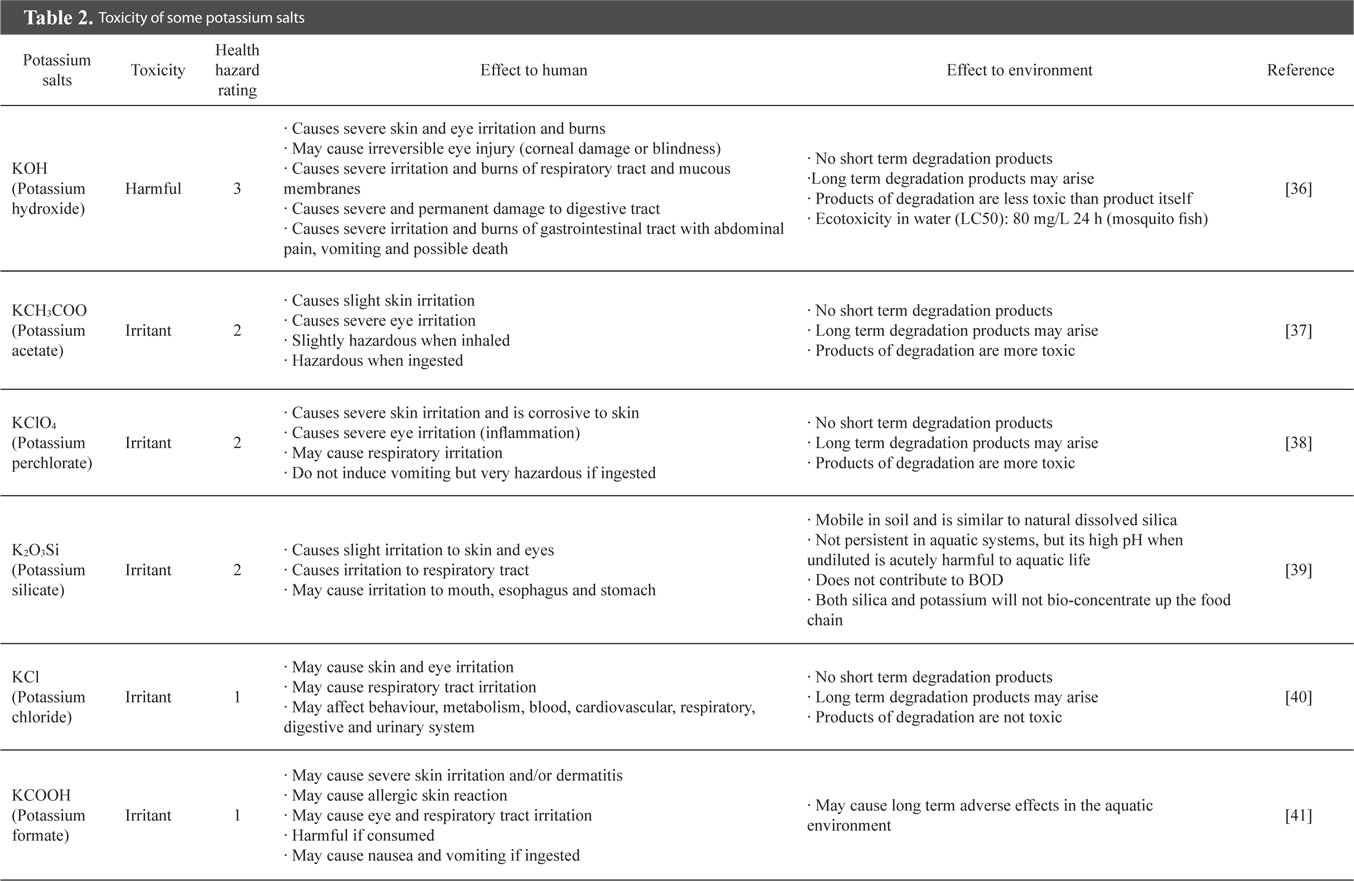
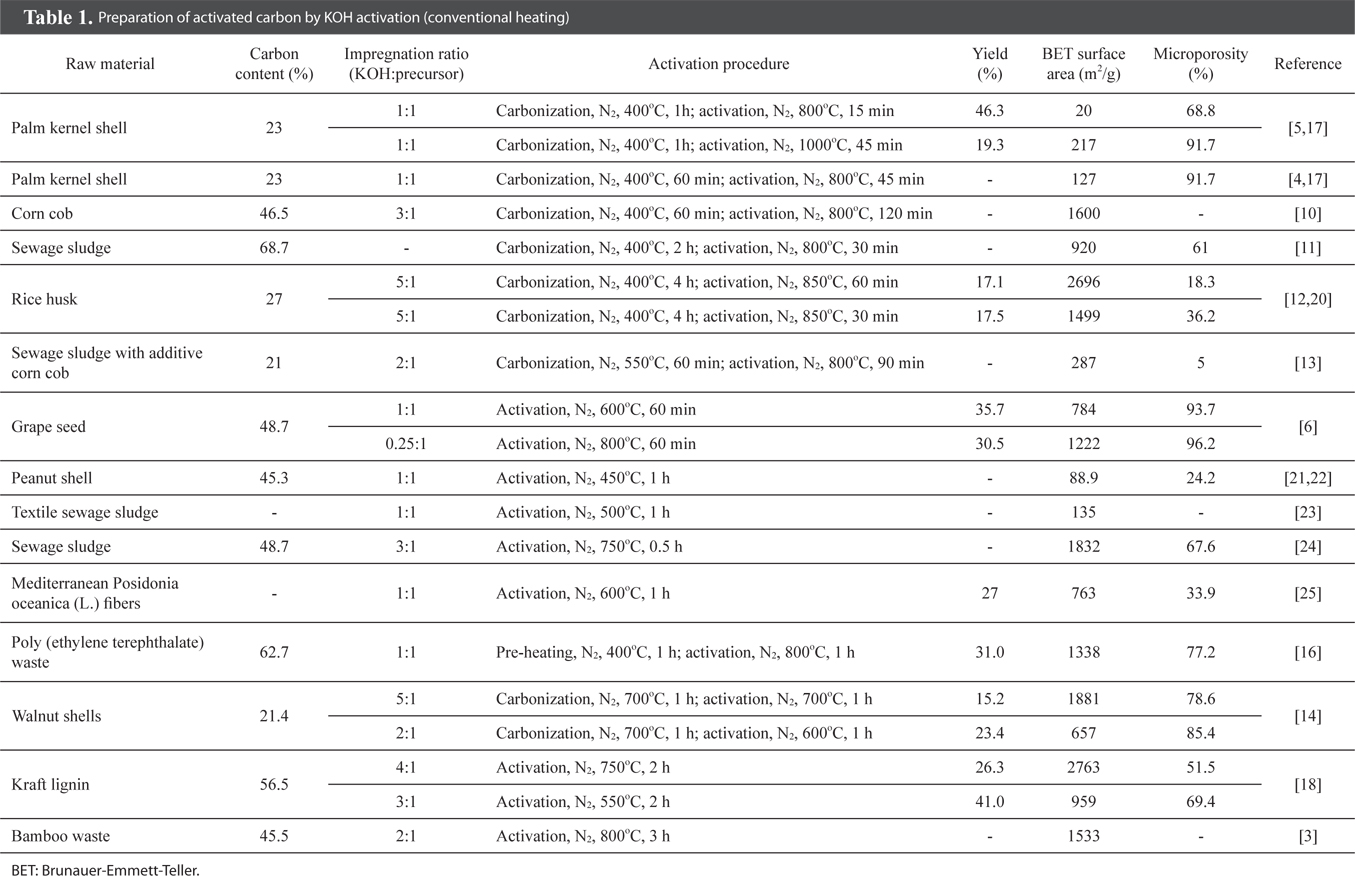
Table 1 exhibits recently developed activated carbon preparation methods using various precursors and KOH activation with conventional heating. From Table 1, it can be seen that char-impregnated chemical activation is more favourable in the manufacture of activated carbon as compared to direct chemical activation. Pre-carbonization, executed in some studies [12,14], produces some advantages in the textural properties of the activated carbon, although KOH is a strong base which supports single step activation.
[Table 1.] Preparation of activated carbon by KOH activation (conventional heating)
Preparation of activated carbon by KOH activation (conventional heating)
According to Zaini and Kamaruddin [1], the need for precarbonization is determined by the chemical nature of the raw material and the activators used. A starting material which undergoes pre-carbonization (in air or N2) could offer larger specific surface area and some initial pore development. As a result, KOH molecules can easily come into contact with the outer surface of the char. Moreover, the organic substances are converted into rich carbon structure which allows the material to be subjected to more KOH activation. In another words, the produced char is more susceptible to chemical reaction with KOH, which consequently leads to the formation of a higher degree of mesopores and micropores. Nevertheless, due to insufficient current information, a distinctive contrast between direct chemical activation and char-impregnated chemical activation is deferred.
From Table 1, it is generally found that an activation temperature above 800℃ and an activation time of more than 30 min will produce activated carbon with a specific surface area greater than 900 m2/g [6,10-12,15,16]. However, some studies have revealed a lower product yield of 17% to 31% when a temperature above 800℃ was used [6,12,17]. In other words, higher activation temperature and longer retention time could promote the further development of pores, which consequently leads to a greater surface area of up to 2696 m2/g, and at the same time suffers a lower carbon yield [12]. The pyrolysis with KOH reduces the amount of carbon due to the intercalation of potassium ions into the carbon matrix, which accelerates the carbon liberation. In addition, further increase in the activation temperature and time may result in both lower surface area and carbon yield, as the micropores collapse to form mesopores and eventually macropores [5].
Li et al. [18] stated that KOH activation of carbon-rich kraft lignin produces activated carbon with the superior surface area of 2763 m2/g. Muniandy et al. [12] also showed that rice husk with 27% carbon content ends up with activated carbon with a similar surface area of 2696 m2/g. Thus, it can be said that the carbon content of the precursor may not necessarily affect the pore development of the resulting activated carbon. However, a carbon-rich precursor is generally a potential candidate for activated carbon.
At present, many studies have focused on furnace improvement for the mass production of activated carbon. Most of the chemically treated commercial activated carbons are being synthesized by the conventional heating method owing to its simple temperature monitoring technique. Rahman and Bari[19] claimed that the production cost of physical activation of rice husk using a fabricated, high capacity furnace is relatively lower than that of a muffle furnace or horizontal tube furnace, while producing activated carbon with better adsorption capacity.
Despite the fact that microwave heating may have some advantages, like shorter activation time, and produces a resultant activated carbon with high specific surface area and yield, scientists and engineers are repeatedly dissuaded by a lack of in-depth understanding of microwave energy especially in the scaling-up of the heating process for mass activated carbon production.
Potassium hydroxide has been widely used as an activating agent in activated carbon preparation. It is evident that KOH activation produces activated carbon with a greater specific surface area and good pore development, but the resulting yield is typically low (around 10%-40%) as compared to other activators like ZnCl2 and H3PO4. This is likely due to the development of pores, which accelerates the carbon loss as a result of the intercalation of metallic potassium ions into the carbon network [26]. During carbonization, KOH acts as a dehydrating agent, to eliminate the presence of water in the precursor, which thereafter would cause the formation of tar that could clog the pores. The carbonization phase is given as:
Dried precursor → Char + Tar + Gasses
During the activation process, the following reactions take place [27,28].
C + 2KOH → 2K + H2 + CO2 C + 2KOH → 2K + H2O + CO CO2 + 2KOH → K2CO3 + H2O
Both mesopores and micropores are formed as a result of the intercalation of potassium into the carbon network during the activation. Besides, there is also a possibility for a secondary reaction to occur as follows [28].
H2O + C + 2KOH → K2CO3 + H2
Abechi et al. [5] stated that potassium carbonate produced from KOH activation could avert excessive sample burn-off, and consequently lead to a high product yield and well developed porosity. However, this notion is not scientifically proven and somewhat contradicted by the findings shown in Table 1. According to Khalil et al. [29], the activated carbon yield is lower at activation temperatures >700℃ because KOH may catalyze the oxidation reactions. As a result, the outer surface carbon atoms are oxidized leading to the formation of pores. This is in agreement with the findings by Mestre et al. [30]. The starting material is likely to disintegrate into powder form when activated with alkaline hydroxide owing to the formation of pores as a result of gasification reactions [31]. In another words, activated carbon with low yield but high porosity is elicited at the expense of char burn-off.
Activated carbon is an economical adsorbent with unique textural properties and good adsorption capacity. In traditional applications, microporous activated carbon (pore width <2 nm) is usually employed. However, the development of highly mesoporous activated carbon (2 nm < pore width <50 nm) has gaining considerable attention due to its wide range of applications.
From Table 1, it is noteworthy that nearly all KOH-activated carbons possess extensive microporosity, and some can reach up to 96%. The grape seed activated carbon is mainly microporous with a surface area of 1222 m2/g [6], while the rice husk activated carbon (2696 m2/g) is mainly mesoporous [12]. The mesoporous activated carbon produced by KOH activation is probably due to the manifestation of the lower carbon content of the precursor, and the high impregnation ratio used in the activation.
In air and wastewater treatment, mesoporous carbon material is more suitable for macro-pollutants removal. For example, methylene blue (cationic dye) molecules are more susceptible to lodge on an adsorbent with a pore diameter larger than 1.5 nm [32]. On the other hand, microporous activated carbon is most widely used, and limited only for the adsorption of micropollutants and heavy metal ions. Because of its small pore diameter (<2 nm), larger size molecules (macro-pollutants) are therefore prevented from entering the pores.
The mesoporous texture commonly aids in the mass transfer of dye molecules into the bulk of the carbon matrix [33]. Hu and Srinivasan [34] investigated the nature of the porosity and adsorption capacity of different molecular size adsorbates. They reported a higher methylene blue (larger molecular size adsorbate) adsorption of 448 mg/g by mesoporous coconut shell activated carbon (Vmes/Vtotal = 71%; specific surface area = 2191 m2/g) [34]. Similarly, Sun et al. [35] reported that activated carbon with a mesoporous ratio of 61% and a specific surface area of 1608 m2/g can attain the high methylene blue uptake of 1012 mg/g. Therefore, it is sufficient to conclude that mesoporous activated carbon is more efficient for the removal of larger adsorbates.
During the selection of the activating agent for activated carbon preparation, toxicity and its implications for the environment and human health ought to be taken into consideration. In KOH activation, the activator, while readily impregnated in the precursor, is not completely vaporized, as the activation temperature is generally lower than the boiling point of KOH (1327℃). Thus, a considerable fraction of KOH is highly expected to be released into the aqueous environment when the activated carbon is washed prior to use. To date, almost none of the published studies highlight concerns over the released effluent containing residual KOH after activation, whether it is in compliance with environmental regulation, or has the potential to be recycled for subsequent activation.
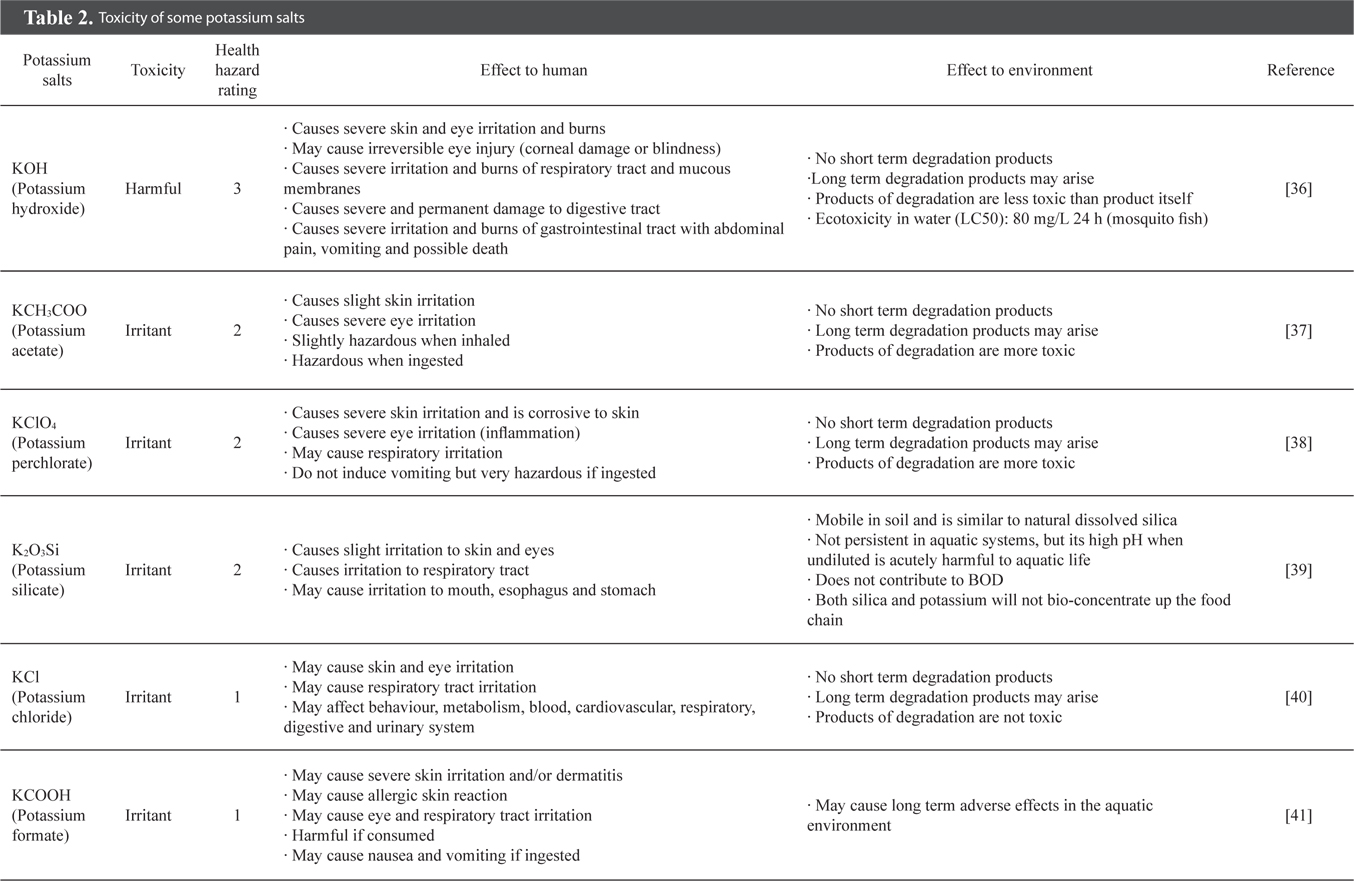
In nature, KOH is poisonous and toxic as it is a strong base chemical. Table 2 shows that KOH has a health hazard rating of 3 which indicates that it is harmful and can cause severe damage to human health [36]. If KOH enters the human body through ingestion, the victim would experience severe and permanent damage to their digestive track, severe irritation and burns of the gastrointestinal tract with abdominal pain and vomiting, and possible death in the worst case scenario [36].
[Table 2.] Toxicity of some potassium salts
Toxicity of some potassium salts
KOH also creates adverse effects in the environment, especially a water body. KOH promotes eco-toxicity in water, although its degradation products are less toxic. The growth of mosquito fish takes place when the eco-toxicity (LC50) for KOH in a water body exceeds 80 mg/L for 24 h [36]. One result is that dengue fever can emerge as a public health problem and may even cause death in serious cases, as there is still no cure for this illness. Besides, the ecological balance in a water body may also be affected due to the sudden rise in mosquito fish population, which consequently affects the entire food chain. Thus, an alternative replacement for KOH as an activator is critically required. Among the family of potassium salts, potassium perchlorate, potassium silicate, potassium chloride and potassium acetate are potential substitutes for KOH owing to their lower toxicity and reduced impact on the environment.
Potassium chloride (KCl) and potassium acetate (KCOOH) could be regarded as the best substitutes for KOH because of their low health hazard rating, and they are safer to handle and store. The implications of both KCl and KCOOH on the human and environment are almost similar. Ingestion of KCl may affect human behaviour, metabolism, blood, cardiovascular, respiratory, digestive and urinary system, while that of KCOOH may trigger nausea and vomiting. The effects of other potassium salts are summarized in Table 2. Nevertheless, further investigations on these alternative activators would be imperative to ensure the effectiveness of activation and also to establish activated carbon with excellent adsorptive properties
Potassium hydroxide (KOH) has been widely used as an activator for preparing activated carbon. Despite producing well developed porosity and a high specific surface area, the activated carbon yield is usually low. Moreover, the activated carbon produced is highly microporous and this somewhat restricts its applications for the removal of macro-pollutants in air and water. There is also considerable concern over the use of KOH as an activating agent because of its toxicity and detrimental impacts on humans and the environment. Such drawbacks should be taken into account in the activated carbon preparation. Also, it is of utmost importance to establish alternative activators in the manufacture of excellent activated carbon.