Pharmacopuncture, or herbal acupuncture, is a modality of treatment that is totally different from traditionally-used methods of Korean medicine. Its therapy is derived from a combination of two traditional therapeutic methods, herbal medicine and acupuncture therapy. Pharmacopuncture treatment is performed by injecting small amounts of herbal medicinal materials at acupuncture points or affected areas in order to benefit from the effects of both herb medicine and acupuncture [1]. In the Korean clinical environment, pharmacopuncture is frequently being used on a daily basis. Nowadays, its safety and efficacy are important issues.
WSGP was prepared in a sterile room at the Korean Pharmacopuncture Institute (Korea-Good Manufacturing Practice, K-GMP).
The animals used in this study were 6-week-old Sprague-Dawley (SD) rats (Orientbio Inc., Korea). The rats were received at an age of 5 weeks, and they were kept for 1 week at room temperature. The mean weights of the rats were 189.5 ─ 209.2 g (male) and 145.1 ─ 167.6 g (female) at the time of injection. For all animals, a visual inspection was conducted; all animals were weighed using a CP3202S system (Sartorius, Germany). During the seven days of acclimatization, the general symptoms of the rats were observed once a day. The weights of the rats were recorded on the last day of acclimatization.
The temperature of the laboratory was 21.0 ─ 23.2°C, and the humidity was 40.9% ─ 59.4%. Enough food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and ultraviolet (UV)-filtered water were provided. The lights were on for 12 hours/day (from 7 am to 7 pm).
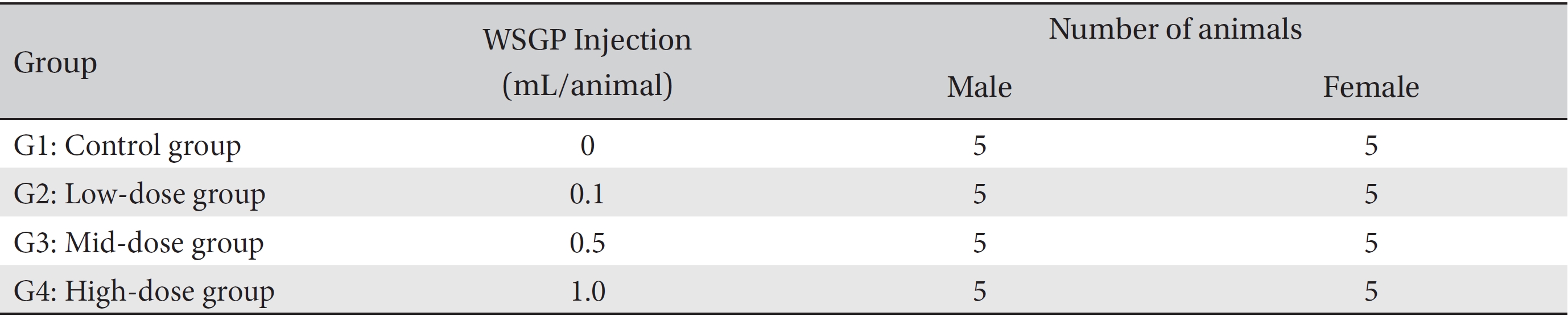
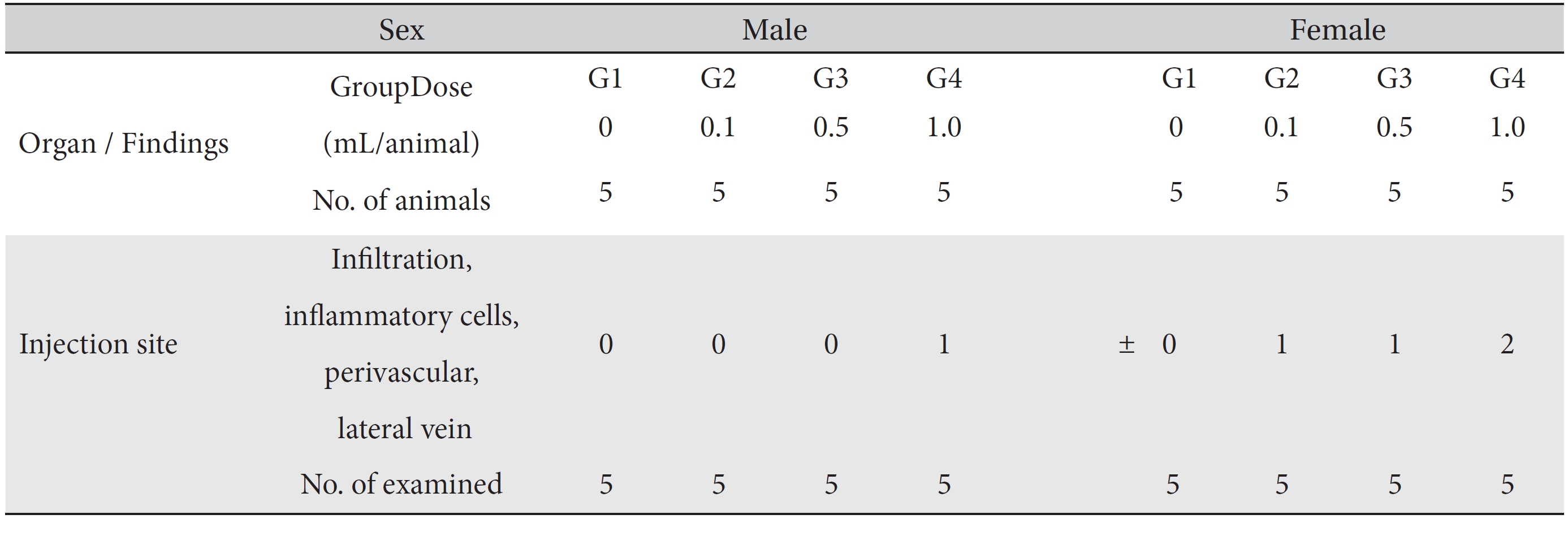
Groupings were done after seven days of acclimatization. Animals were selected if their weights were close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were randomly distributed into 4 groups (5 male and 5 female rats per group, Table 1).
The expected volume of WSGP in clinical use is 1.0 mL per treatment. No deaths occurred in a pilot test (Biotoxtech Study No. B12887P), so based on that study, 1.0 mL of WSGP pharmacopuncture was injected into each male and female rat in the high-dose group. The administered volume of WSGP was 0.5 mL/animal in the mid-dose group and 0.1 mL/animal in the low-dose group. One mL/ animal of normal saline was administered to the rats in the control group. The administration route was intravenous because of clinical considerations. The syringe used in the experiment was a 1-mL disposable 26G syringe. The WSGP was injected at a speed of 2 mL/minutes through the tail vein. This study was conducted under the approval of the Institutional Animal Ethics Committee of Biotoxtech (No. 120896).
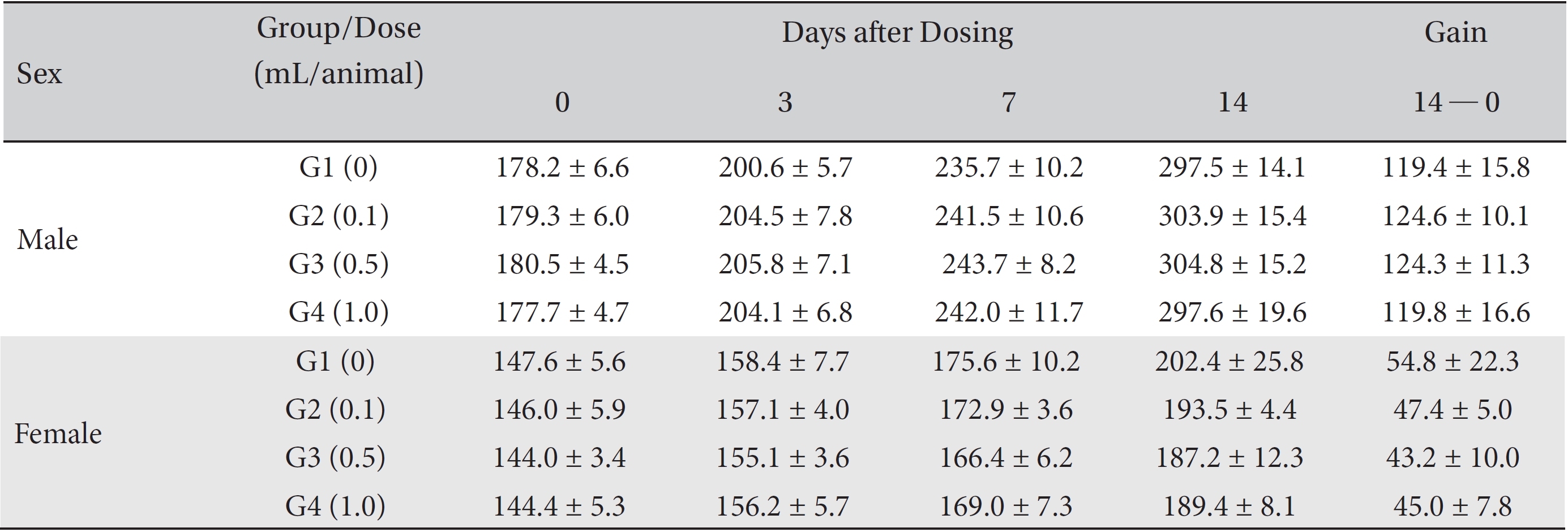
From the 1st day to the 14th day of treatment, the general symptoms were examined once a day. On the day of injection (day 0), the general symptoms (toxicological effects, manifestation time, recovery time, etc.), as well as mortalities, were examined at 30 minutes and 1, 2, 4, and 6 hours after injection. Body weights were measured immediately before treatment and at 3, 7 and 14 days after treatment.
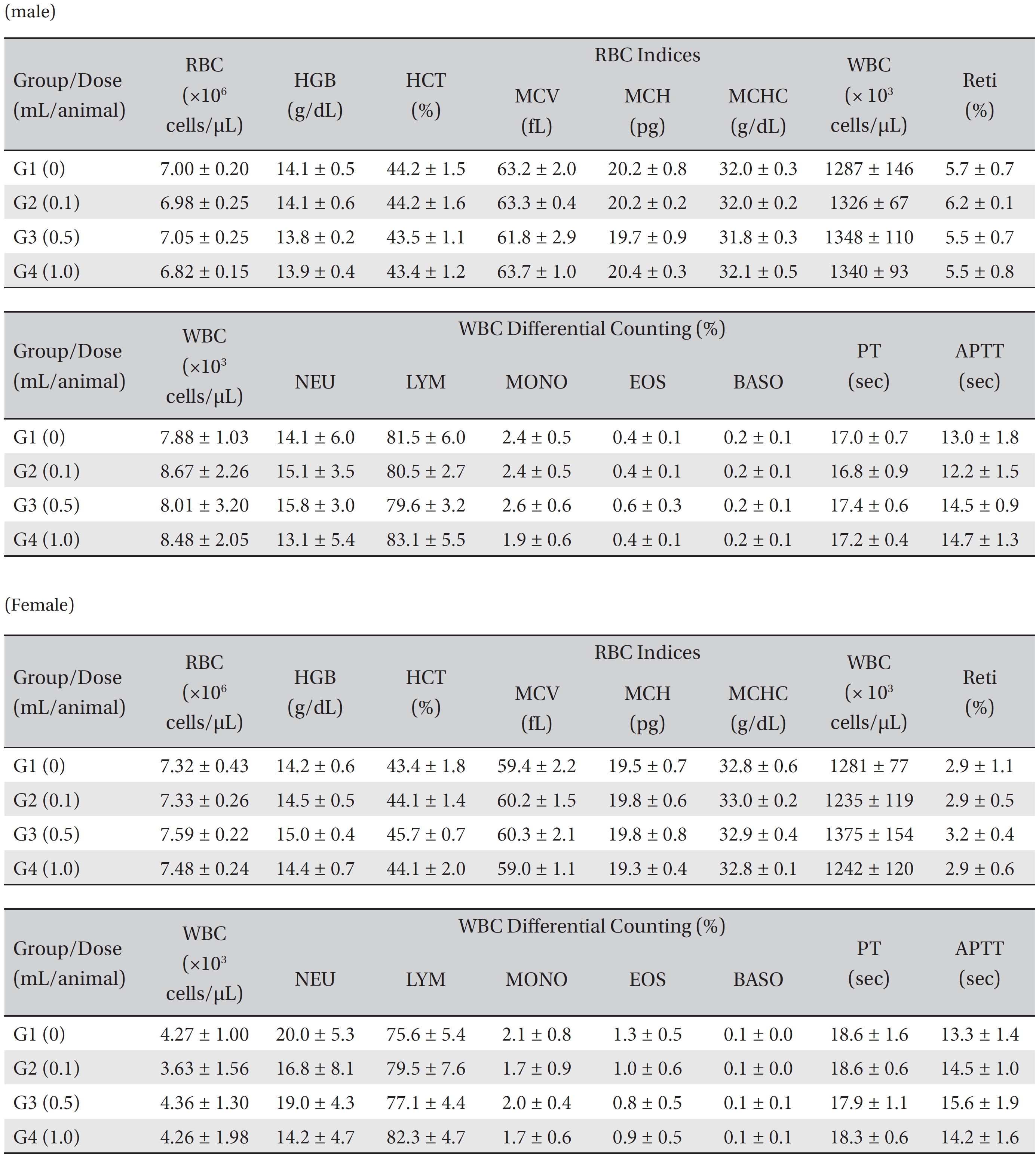
After the rats had fasted for more than 18 hours after the treatment had been completed, they were anesthetized in a chamber (V-1 tabletop lab animal system, U.S.A.) filled with isoflurane gas. A blood sample was taken from the abdominal aorta on the necropsy day (15 days after injection) and was inserted into an ethylenediaminetetra–acetic-acid (EDTA)-coated tube. The 1 mL of blood was analyzed by using an automatic hematology analyzer (ADVIA 120, SIEMENS, Germany). The items measured were erythrocytes (RBC), hemoglobin, hematocrits, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), leucocytes (WBC), WBC differential counting (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), and reticulocytes. A 2.0-mL blood sample was centrifugated for the blood coagulation test (3,000 rpm, 10 minutes), and serum was taken. The results were measured by using an automated coagulation analyzer (Coapresta 2000, SEKISUI, Japan). The items measured were the prothrombin time (PT) and the activated partial thromboplastin time (APTT).
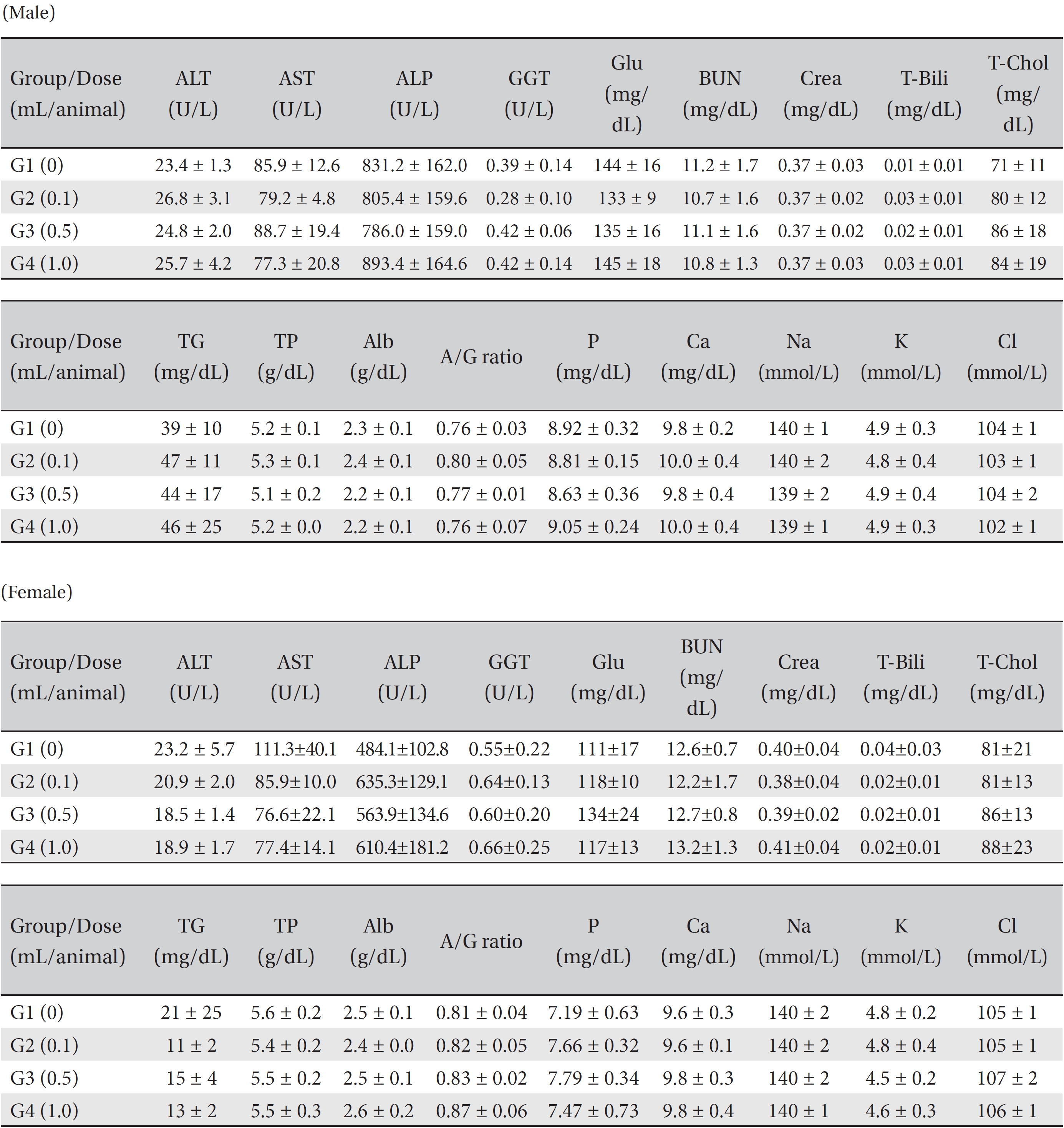
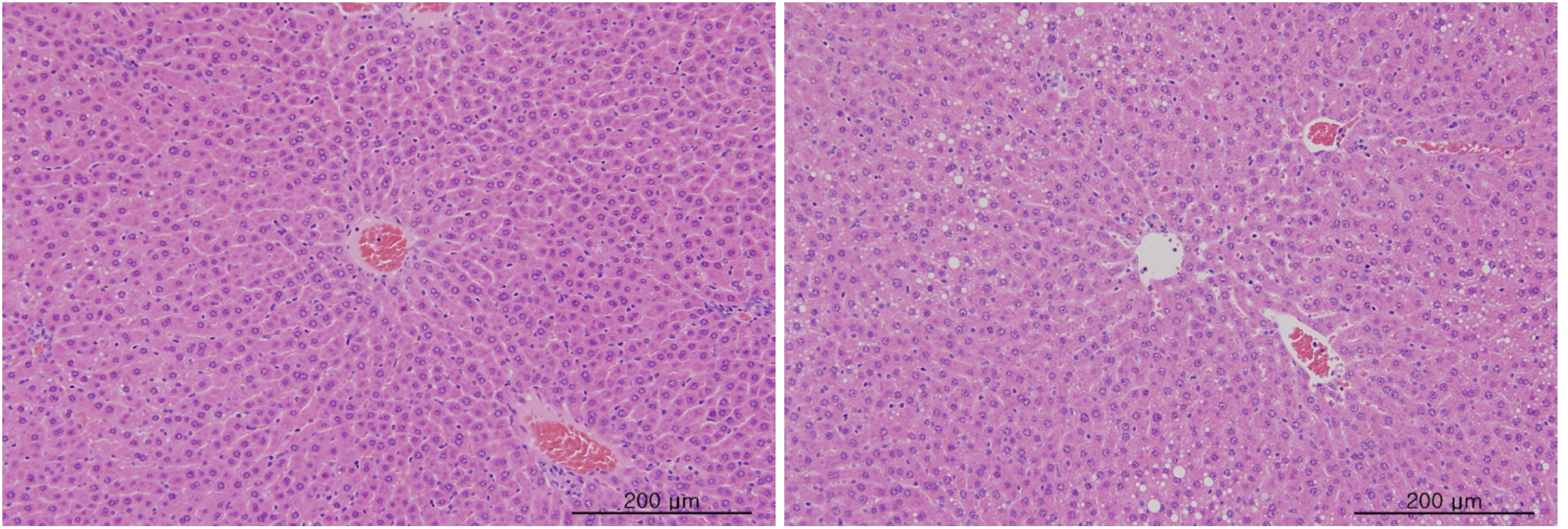
Blood taken from the abdominal aorta was used in the blood biochemical test. The results were measured by using an automatic analyzer (7180, HITACHI, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany). The items measured were alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), albumin/globulin ratio (A/G ratio), total cholesterol (T-Chol), triglyceride (TG), phosphate (P), glucose (Glu), calcium (Ca), chloride (Cl) and potassium (K). After the termination of all observations, organs and tissues of all animals were visually inspected and were examined under a microscope after they had been stabilized by using 10% neutral buffered formalin. For the injection site, tissue slices were stained with hematoxylin & eosin (H&E).
The body weights and the results from the hematologic examinations and the blood biochemical tests were analyzed by using statistical analysis system (SAS) software (version 9.3, SAS Institute Inc., U.S.A.). The Bartlett test was conducted to evaluate the homogeneity of the variance and the significance. The significance level was 0.05. The one-way analysis of variance (ANOVA) test was conducted, and when the homogeneity of the variance was recognized, Dunnett’s
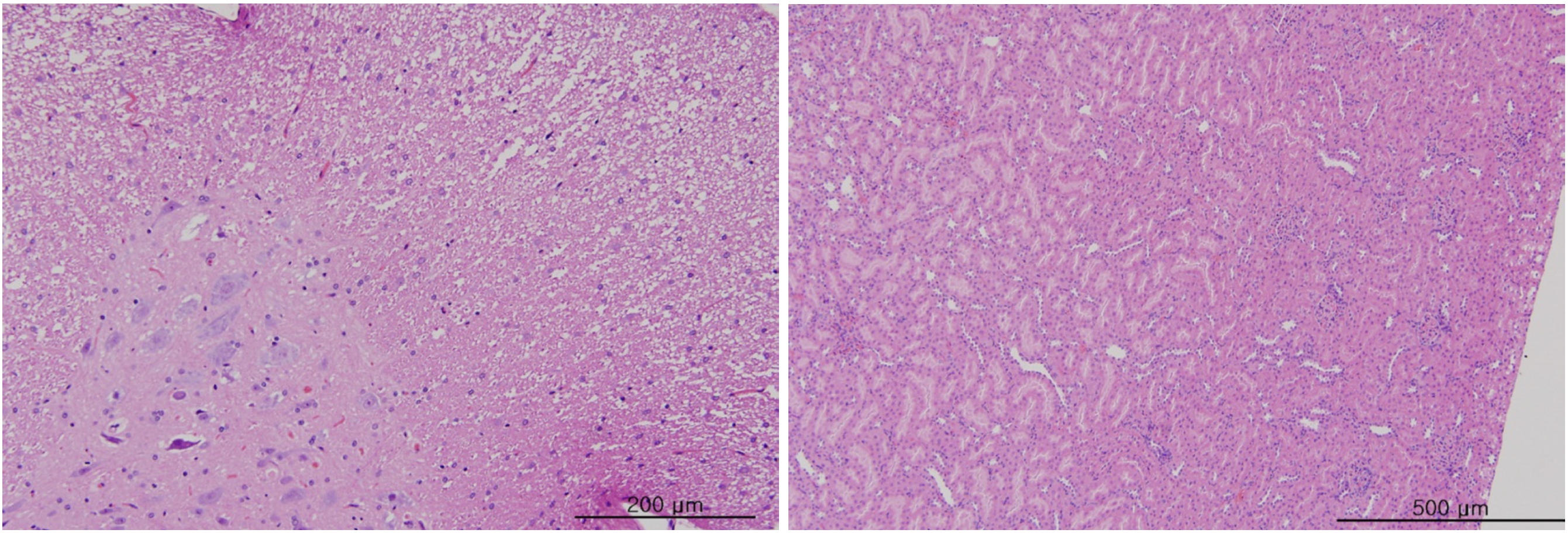
In this study, no deaths occurred in either sex during the experiment, and no meaningful changes in body weights or abnormalities in the rats' general conditions were noticed (Table 2). No meaningful changes in the hematological and the biochemistry tests were found. (Tables 3,4). Also, the necropsy findings showed no abnormalities, however perivascular infiltration of inflammatory cells into the lateral vein at the injection site was observed in the male high-dose group and in the female low-dose, mid-dose and hige-dose group, but those changes were minimal and seemed to be due to the injection (Table 5, Figs. 1,2,3,4).
Although Ginseng (
The results of administering WSGP via a venous route did not cause any changes in weight development, in the hematological and the biochemical tests or in the necropsy findings. In addition, no deaths were observed. These results indicate that venous administration of WSGP is a safe modality of treatment.
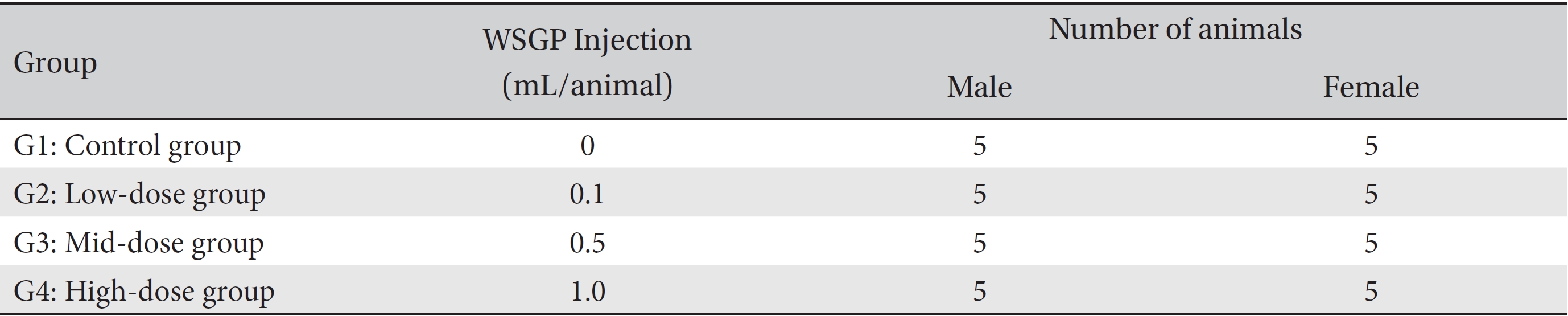
Groups of animals
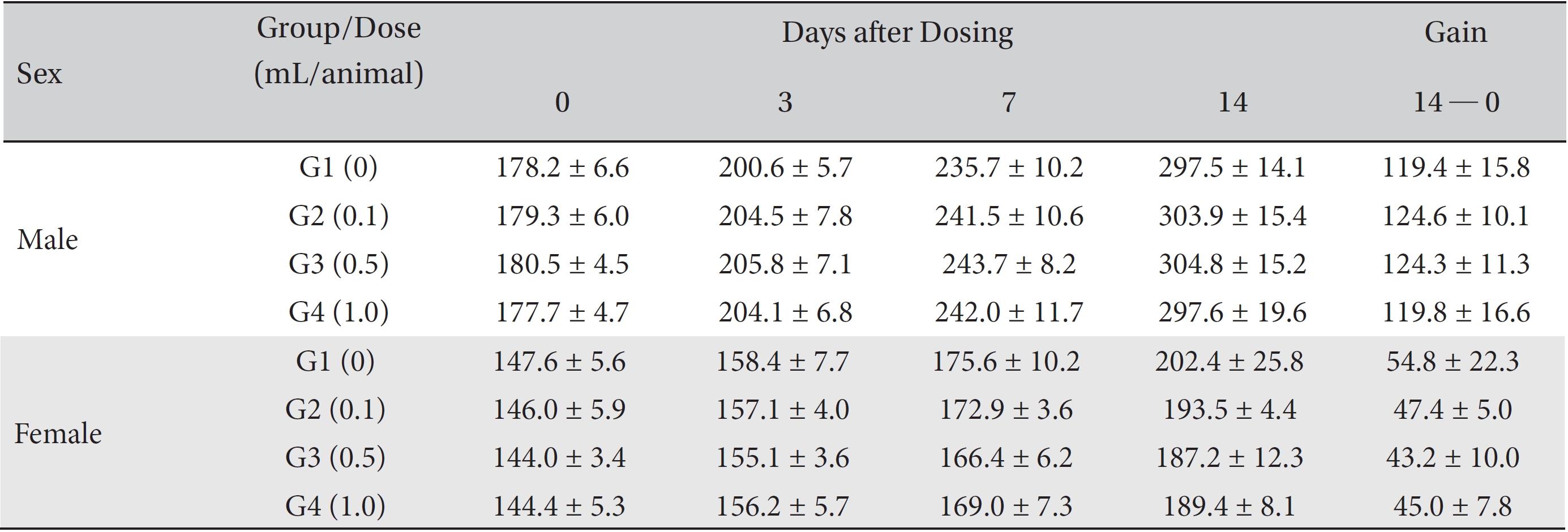
Mean body weights
[Table. 3] Mean hematology parameters in male, female SD rats
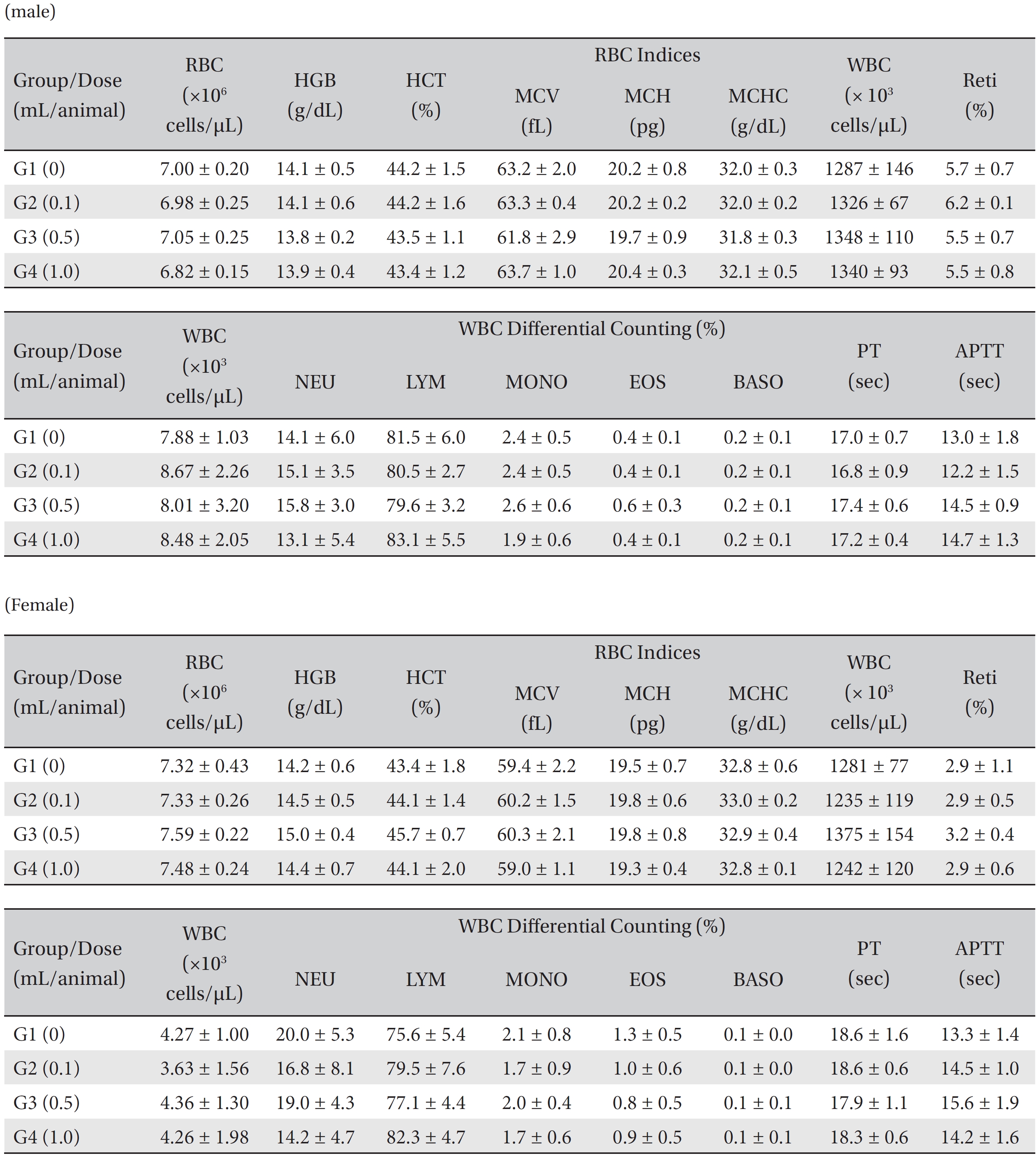
Mean hematology parameters in male, female SD rats
[Table. 4] Mean clinical chemistry in male, female SD rats
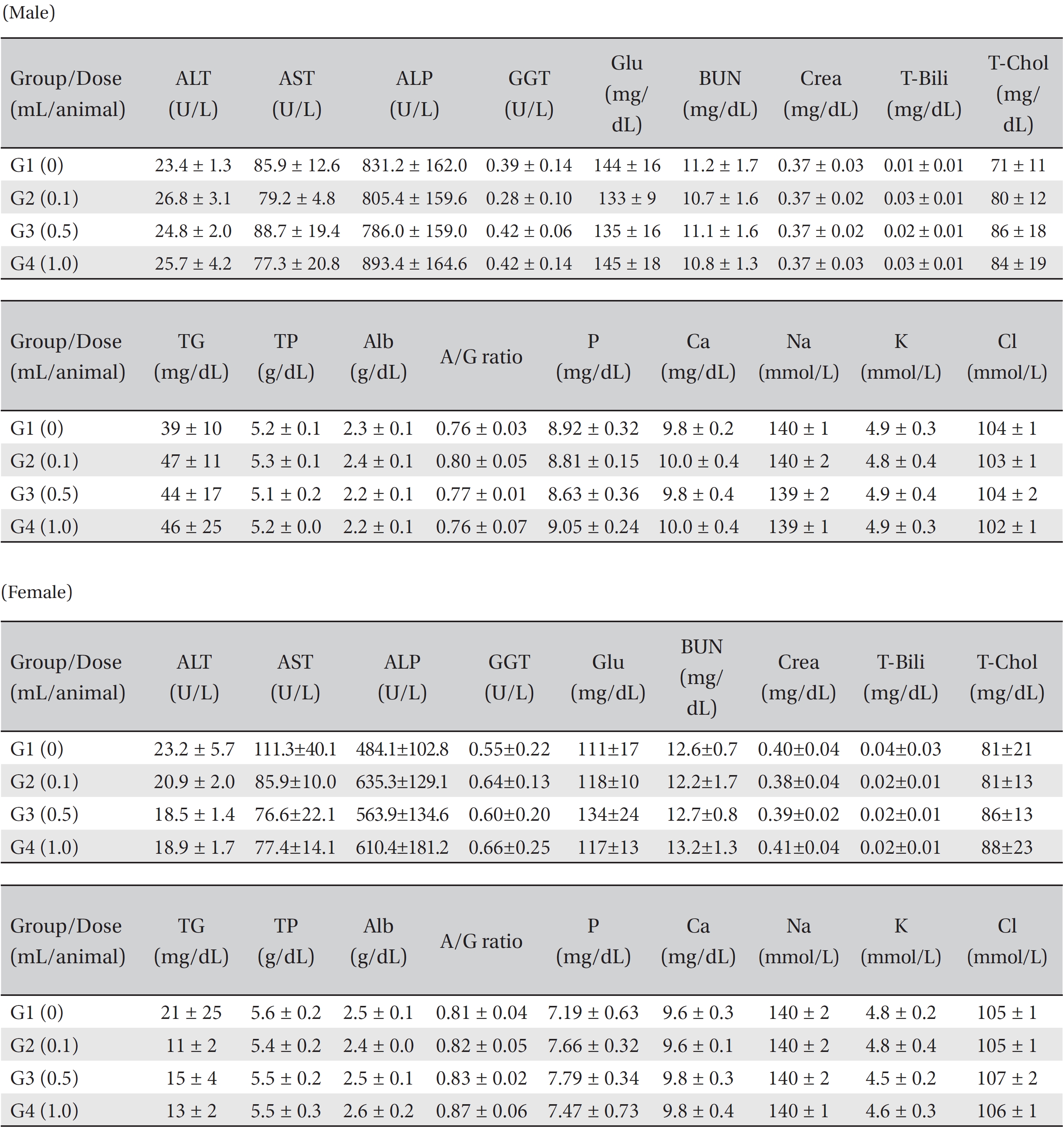
Mean clinical chemistry in male, female SD rats
[Table. 5] Summary of histopathological findings
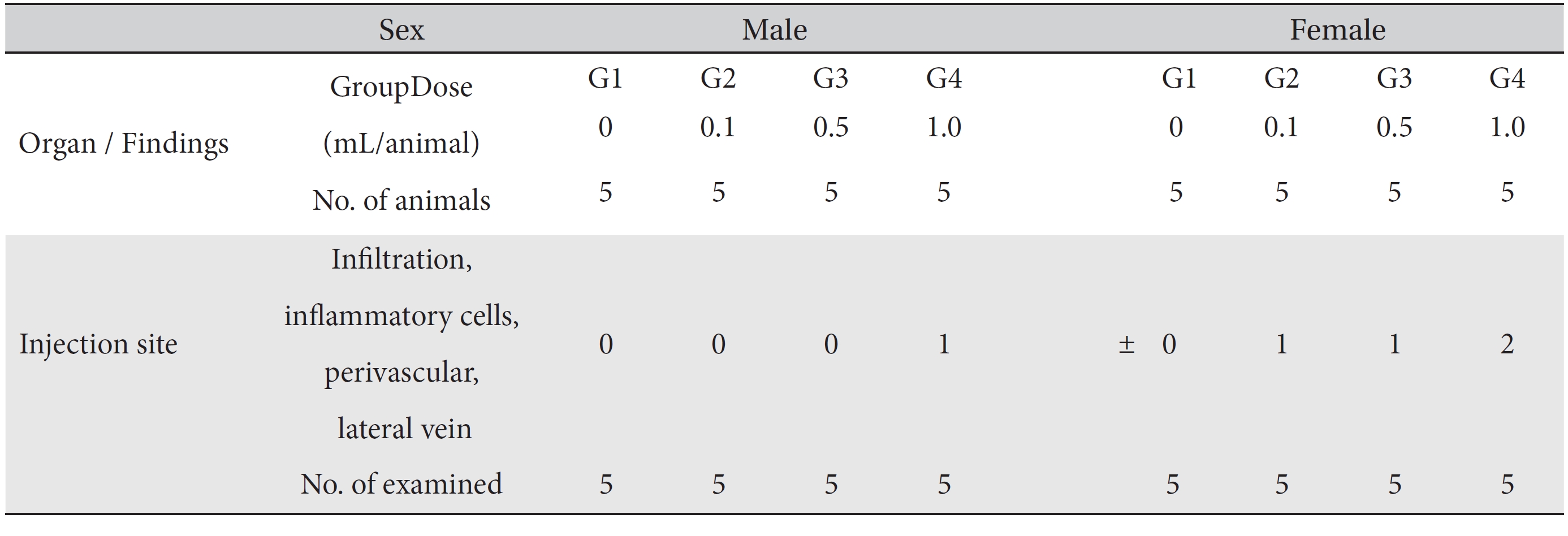
Summary of histopathological findings