Lake sediments, as a major internal pollutant, are generated from various nutrients such as nitrogen, phosphorus and substances such as heavy metals and pesticides by changing their states from dissolved phase into particular phase, and also generated by attaching to substances and descending in the water column. Even though these sediments are subject to contaminant removal as they sorb pollutants in the water body, mostly they remain continuous pollutant sources in water systems due to contaminant release reactions (Hakanson and Janson 1983). The amount and duration of contaminant release from the upper layer of the sediments depends on the sediment composition, microbial activities, surrounding environment, present state of pollutants, and various other factors. If the pollutant substances are present in a form that is easily dissolved, pollution of the upper water column is increasingly likely (Oh and Cho 2007). Phosphorus, which is usually stored in sediments according to anaerothe water body’s material cycle, is one of the most important nutrients that determine the primary production rate of an aquatic ecosystem. Therefore, excessive phosphorus release causes problems that stimulate lake eutrophication (Dorich et al. 1984).
Phosphorus in sediments may be released into the water body via several environmental changes, such as water temperature, pH and dissolved oxygen (DO), and the released pollutants influence the nutritional state of the lake, which stimulates eutrophication. Hence, DO varies easily in the lake and is also considered a significant environmental factor (Mortimer 1971, Chapra and Canale 1991). However, this factor has been considered generally unrelated to phosphorus release. Although many studies have reported that phosphorus release action is generally activated in an anaerobic state, a few papers have reported that phosphorus release and sorption occur regardless of anaerobic and aerobic conditions (Furumai and Ohgaki 1989). In addition, Holdren and Armstrong (1980) reported that since not all phosphorus can be released from sediment and that phosphorus in sediment is present in a form both easily released or not easily released according to the lake environment, it is necessary to analyze the forms of phosphorus in sediment in order to accurately evaluate the phosphorus release characteristics and possibility.
Generally, the forms of phosphorus are divided into organic phosphorus and inorganic phosphorus, each of which can be further subdivided according to the composition rate of minerals or the organic rate, etc. Kovar and Pierzynski(2009) classified the forms of phosphorus into five stages for inorganic phosphorus, namely, loosely and soluble-P, Al-P, Fe-P, reductant soluble-P, and four stages for organic phosphorus, namely, labile-P, moderately labile-P, humic/fulvic acid-P and non-labile-P. Each subsequent phosphorus stage has stronger bonding and is therefore more difficult to be released into the water body and less affected by the impact of environmental changes.
In this study, we experimentally evaluated the behavioral characteristics of phosphorus in aerobic and anaerobic conditions with sediments of Hwaseong Lake. We also analyzed the forms of phosphorus with the samples before and after the previous experiment to understand the major forms of phosphorus related to the release and sorption.
>
Sediments sampling and analysis
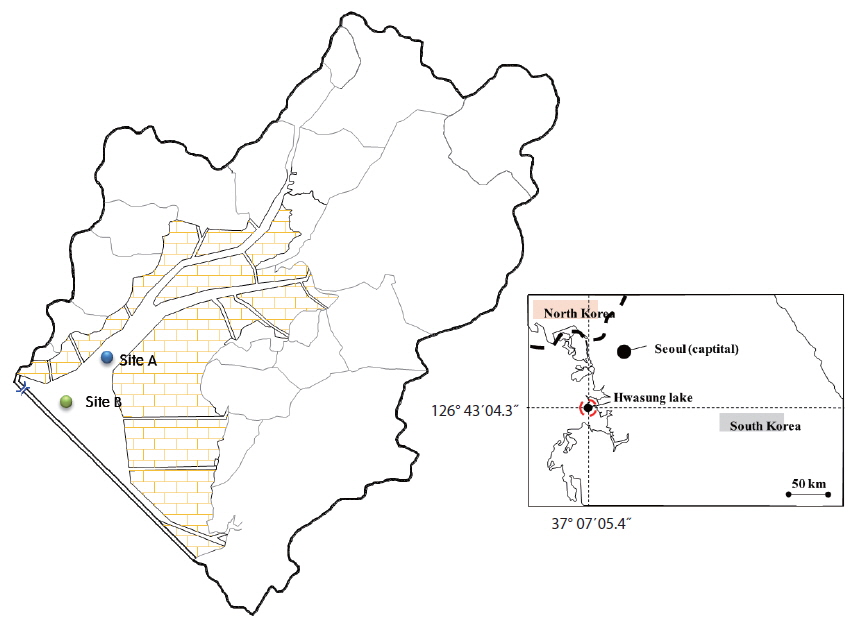
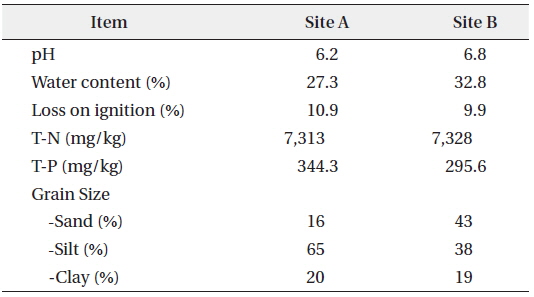
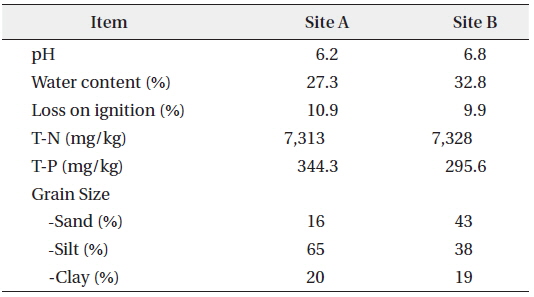
The sediments used in this study were sampled from Hwaseong Lake, located in Gyeonggi-do, Republic of Korea. Hwaseong Lake, made by damming Namyang-bay with a tide embankment, is an artificial lake with an area of 1,730 ha and volume of 54,600,000 m3. Desalination work has been recently conducted to secure agricultural water supply use for farmlands in reclaimed land and hinter land (Kang and Jang 2015). The sediment sampling sites were Site A, where two rivers flow in and mixed thoroughly, and Site B, located in a straight flow line from Site A and affected by a sea dike sluice (Fig. 1). A Grab Sampler was used for sediment sample collection. The sediments samples for behavioral characteristic analysis were dried at room temperature until constant weight and then ground to pass a 2 mm sieve (MERK 2002). Sediments pH of Sites A and B were measured in a 1: 5 soil-to-water suspension after shaking for 2 hours. Water content and loss on ignition measurements were based on weight losses after drying and combustion of the sediments at 105 and 550 ℃, respectively. Total phosphorus in the sediments was measured by perchloric acid (HClO4) digestion method (Jackson 1958) and total nitrogen was measured by modified Kjeldahl method (KS I ISO 2008). Sieve analysis and pipette method were used to determine the grain size distribution (Gee and Bauder 1986). The results are shown in Table 1.
[Table 1.] Physical and chemical analyses of the sediments used
Physical and chemical analyses of the sediments used
>
Method to evaluate the behavioral characteristics of phosphorus (P)
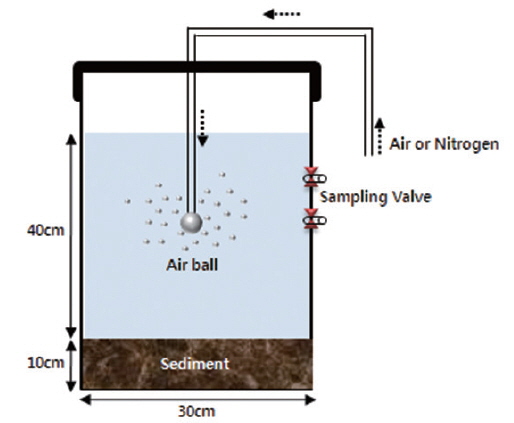
The behavioral characteristics experiment was conducted with a cylindrical reactor made of acrylic material, with a range of DO concentration from aerobic to anaerobic conditions. To generate aerobic conditions, O2 gas was injected to increase DO concentration in the experimental apparatus to a constant 9~10 mg/L. For anaerobic conditions, N2 gas was injected continually under sealed condition to prevent oxygen ingress into the experimental equipment (Fig. 2). In addition, light was blocked to prevent photosynthesis change of oxygen conditions by covering the whole reactor with aluminum foil for the duration of the experiment. These gas injection conditions were continued for 120 hours, after which the sediments in the reactor were sampled to analyze total phosphorus concentration and the forms of phosphorus.
>
Forms of phosphorus analysis
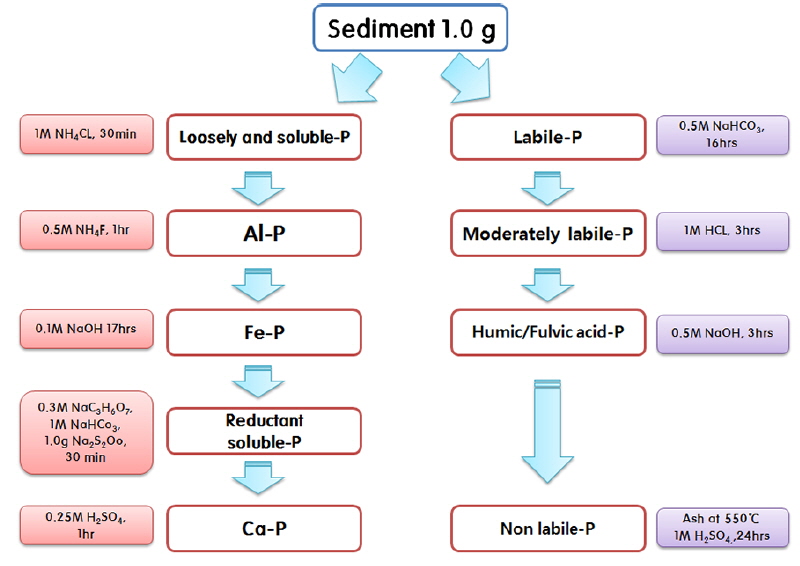
The forms of phosphorus were analyzed according to the organic phosphorus and inorganic phosphorus by using the sequential extraction method (Kovar and Pierzynski 2009). Specific experimental information is provided in the mimetic diagram shown in Fig. 3.
One gram of sediment sample for each organic phosphorus and inorganic phosphorus experiments was placed in a 50 mL centrifuge tube, the proper reagent was injected for each step and released using a shaker, the supernatant was separated with a centrifugal filter (ESV-180C; E-Science, Seoul, Korea) and the phosphorus concentration was measured through an ascorbic acid test with UV spectrophotometer (Optizen POP; Mecasys, Daejeon, Korea).
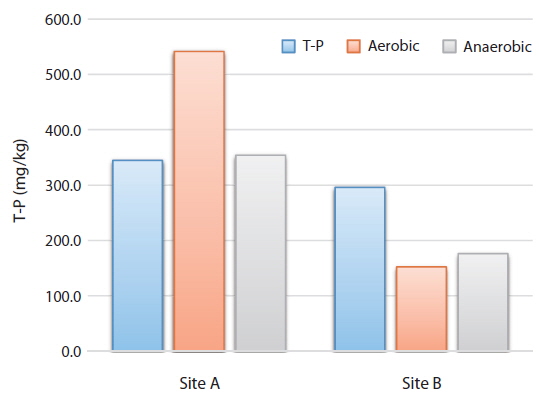
From the analysis of the changes of the total phosphorus concentration of the sediments of Sites A and B at different DO conditions (aerobic and anaerobic) with 120 hour artificial gas injection of the sediments, the phosphorus sediment concentration at Site A increased by 57% from 344.4 mg/kg pre-experiment to 541.2 mg/kg post-experiment under aerobic conditions and by 2.7% to 353.8 mg/kg under anaerobic conditions. In contrast, the phosphorus concentration at Site B decreased from 295.6 mg/kg to 152 mg/kg and 176 mg/kg, respectively.
Consequently, the aqueous phosphorus was sorbed into the sediments of Site A, but released into the water body from the sediments of Site B (Fig. 4). In contrast to our study finding, many studies related to the behavioral characteristics of phosphorus have generally shown that the phosphorus release rate increased in anaerobic condition (Bostrom 1988). This shows that the phosphorus release does not totally depend on the environment. Holdren and Armstrong (1980) reported the significance of the forms of phosphorus in sediment on release.
>
Inorganic Phosphorus presence
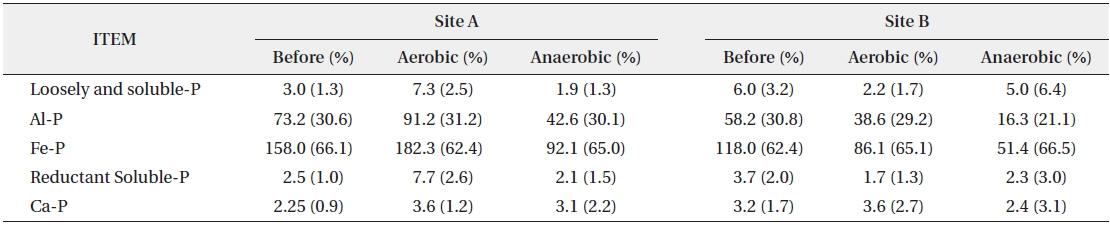
Among the inorganic phosphorus forms, loosely and soluble-P are electrically adsorbed in the surface layer of sediments or dissolved in the pore water of sediments, resulting in weak cohesion. Consequently, changes in the physical environment such as sediment disturbance, DO, temperature and phosphorus concentrations allow the inorganic phosphorus to transfer easily into the water body (Kaiserli et al. 2002). However, in this study, the initial concentrations of loosely and soluble-P in the sediment of Site A and B were low at 3.0 mg/kg and 6.0 mg/kg (Table 2) respectively, and did not vary significantly following experimental change in the DO concentration.
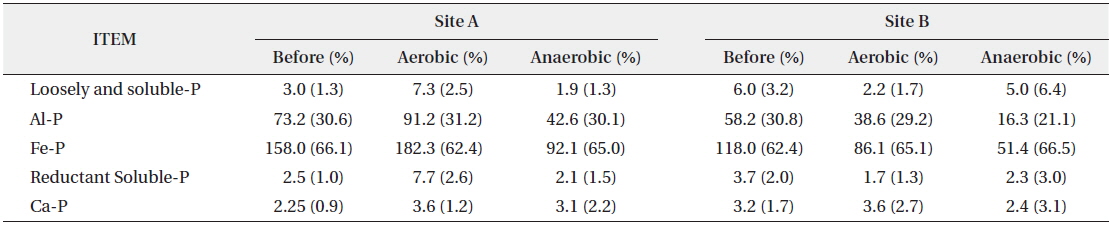
Change of inorganic phosphorous forms according to dissolved oxygen (DO) condition (unit: mg/kg)
Al-P and Fe-P form a complex with Al or Fe and are precipitated or co-precipitated as Fe2O3 and Al(OH)3, etc. If the oxidation-reduction potential decreases or pH increases, they are released into the water body. Since greater amounts of Al-P and Fe-P are released than other phosphorus forms, they play an important role in the material circulation between the sediments and the water body (Bores 1991, Lee et al. 1995, Zhou et al. 2001). In this study, Al-P and Fe-P concentrations were the highest, suggesting that most of the forms of inorganic phosphorus are combined with Al and Fe. Other studies showed similar results (Ishio et al. 1986, Kaiserli et al. 2002). At Site A, two forms of phosphorus (Al-P and Fe-P) were increased in aerobic condition and decreased in anaerobic condition, whereas both forms were decreased in both aerobic and anaerobic conditions at Site B. Therefore, Al-P and Fe-P among the inorganic forms of phosphorus were relatively more affected by anaerobic condition.
Reductant-P, in which phosphorus is stuck to the Mn complex body, is sensitive to oxidizing reaction and easily released if pH increases (Kozerski and Kleeberg 1998). Ca-P forms minerals like apatite and Ca-containing phosphorus materials, and is difficult to be released (Lee et al. 1995). In this study, the concentrations of reductant- P and Ca-P were low, and the phosphorus concentration did not change much after the experiment.
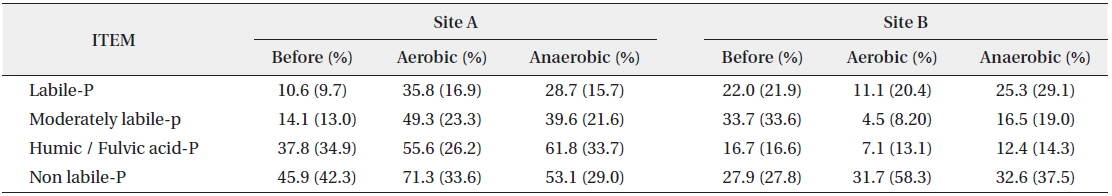
Organic phosphorus cannot directly be used by microorganisms, but when present in sediment, it can potentially be available for use by microorganisms through decomposition (Zhang et al. 2008). In the organic forms of phosphorus, at Site A, humic/fulvic acid-P and non-labile-P showed high concentration at 37.82 mg/kg and 45.86 mg/kg respectively. All forms of phosphorus increased in both aerobic and anaerobic conditions after the behavioral characteristics experiment according to the oxygen condition. At Site B, moderately labile-P was highest at 33.7 mg/kg, followed by non-labile-P at 27.9 mg/L. In the behavioral characteristics in aerobic condition, except for non-labile-P, the concentrations of all forms of phosphorus decreased, whereas in anaerobic condition, all decreased except for increases of labile-P and non-labile-P by 3.3 mg/kg and 4.7 mg/kg, respectively. Especially, moderately labile-P decreased markedly in both aerobic and anaerobic conditions (Table 3).
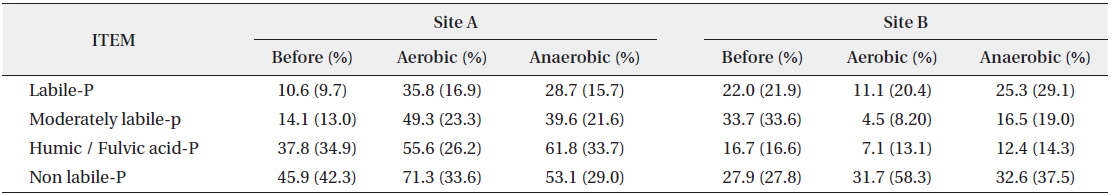
Change of organic phosphorous forms according to dissolved oxygen (DO) condition (unit: mg/kg)
Consequently, for the inorganic phosphorus, the Al-P and Fe-P forms were present in most fractions, and can therefore be expected to be the major forms of inorganic phosphorus. They were also released and/or adsorbed easily responding to environmental changes. On the other hand, organic phosphorus concentration was significantly changed after the behavioral characteristics experiment, which indicated that the organic forms of phosphorus are relatively significant impact factors on phosphorus behavioral characteristics between water body and sediments.
In the inorganic forms of phosphorus before and after the behavioral characteristics experiment, the overall concentration was changed but the composition ratio of each form was maintained at a similar level (Table 2). This indicated that inorganic phosphorus in sediment is not influenced by its composition forms but its concentration is changed due to environmental condition changes such as DO. This suggests that inorganic phosphorus is easily affected by the change of DO conditions and the forms of inorganic phosphorus are not very influential. However, further studies are necessary to confirm this finding because of the limited number of samples in the present study.
In contrast, for each form of organic phosphorus, the change of the ratio was more significant than inorganic phosphorus after the experiment of the behavioral characteristics (Table 3). The behavioral characteristics of the total phosphorus were increased in both aerobic and anaerobic conditions at Site A and showed a tendency of sorption with sediment in both conditions, but the sorption degree was greater than anaerobic condition in the aerobic condition. At Site B, the total phosphorus tended to show release characteristic from sediment in both conditions. This suggests that sorption/release characteristics of organic phosphorus in the sediment were partially influenced by the aerobic and anaerobic conditions, but fundamentally, they were more affected by the forms of organic phosphorus. In fact, in the forms of phosphorus present in the sediments of Site A, the concentration and ratio of humic/fulvic acid-P and non-labile-P, which are difficult to release, were high, whereas the ratio and concentration of labile-P and moderately labile-P, which are relatively easy to release, were low. In general, some of the phosphorus was released into water body due to the disintegration reaction of organic compounds (Kim and Kim 2002, Zhang et al. 2008), and they were released in sequence of easy to be disintegrated.
The sediment at Site A contained less organic phosphorus, which is easily decomposed, but greater amounts of phosphorus, which is strongly attached. Therefore, the sorbed amount was greater than the release rate and the overall phosphorus concentration in the sediment was increased. In contrast, the sediment in Site B contained higher ratios of labile-P and moderately labile-P, which are relatively easy to transfer into the sediment. In other words, the easily released form of organic phosphorus at site B was discharged into the water body, so the overall phosphorus concentration in the sediment was decreased.
The ratio of phosphorus present in the sediment after the behavioral characteristics experiment showed a clear tendency. The ratio of inorganic phosphorus forms in the sediments of Sites A and B were maintained regularly after the experiment. On the other hand, the ratio of humic/fulvic acid-P and non-labile-P in organic phosphorus, which were forms with relatively strong coherence in the sediment of Site A, were greatly decreased, and the ratios of labile-P and moderately labile-P, which were forms with weak coherence, were rather increased. Considering the fact that the sorbing action at Site A was superior since the phosphorus concentration in the sediment increased after the behavioral characteristics experiment, a large amount of phosphorus in the forms of labile-P and moderately labile-P flowed into the sediment.
Considering that a large amount of phosphorus at Site B was released from the sediment into the water after the behavioral characteristics experiment, the ratios of humic/fulvic acid-P and non-labile-P in organic phosphorus, which are difficult to release, were increased, although their relative abundances were changed, and the ratio of moderately labile-P form greatly decreased, which contributed to major release action. Based on this result, considering the phosphorus release from sediments according to the change of oxygen condition, inorganic phosphorus contributed strongly because of its large amount, but the forms of organic phosphorus present in the sediment cannot be ignored either. Especially, labile-P and moderately labile-P in organic phosphorus contributed greatly to the phosphorus concentration change in the sediment by sorption and release reaction.
Phosphorus, known as a major cause of lake eutrophication, affects the lake water composition through the release and sorption with sediments. From the experimental results, the phosphorus concentration increased from 344 mg/kg pre-experiment to 541 mg/kg post-experiment under aerobic condition and to 353.8 mg/kg under anaerobic condition at Site A, indicating that sorption action by sediments was superior, but decreased from 295.6 mg/kg to 152 mg/kg and 176 mg/kg, respectively, at Site B, indicating that release action was superior. This suggests that aerobic and anaerobic conditions do not necessarily affect the behavioral characteristics of phosphorus.
In the study of the forms of inorganic phosphorus, Al-P and Fe-P concentrations were the highest, and were therefore considered the most important forms. In the experimental investigation of the inorganic forms of phosphorus in the sediment according to the oxygen condition, the forms of inorganic phosphorus did not greatly affect the sorption or release reaction because the distribution ratios of the inorganic forms remained constant corresponding to the change of oxygen conditions.
Among the forms of organic phosphorus at Site A, the concentrations of humic/fulvic acid-P and non-labile-P, which are a relatively stronger combination, were highest. At Site B, the concentrations of labile-P and moderately labile-P, which are a relatively weaker combination, were highest and each case influenced both sorption and release. In addition, as a result of changes in the ratio of each form after the experiment, the ratio of labile-P and moderately labile-P at Site A greatly increased, which was judged to mainly contribute to sorption in sediment. In contrast, the amount of phosphorus at Site B decreased in the sediment and the ratio of other forms of phosphorus increased, whereas the ratio of moderately labile-P greatly decreased. Therefore labile-P and moderately labile-P among the organic phosphorus forms were the major mechanisms of sorption in sediment of Site A and moderately labile-P was the greatest contributor to phosphorus release action in sediment of Site B.
In terms of the impacts on the behavioral characteristics of phosphorus in sediment, these study results suggest that the presence of phosphorus, as well as environmental changes, is also a very important factor. Therefore, both phosphorus presence and changes in environmental factors will need to be considered in future analysis of sediments for controlling internal contamination of reservoirs.