Spring-flowering ephemerals of northern hardwood forests have a specific life-history trait; shoot growth and seed production occur simultaneously during a period from snowmelt to canopy closure (Schemske et al. 1978, Nault and Gagnon 1993, Gutjahr and Lapointe 2008). Because of a relatively short epigeous growth period (40-60 days), most of these species are clonal and perennial (Lapointe 2001, Whigham 2004).One way to cope with a short life cycle is early-season growth. This strategy means potentially high risk of encountering weather conditions unsuitable for plant growth and for pollinator activity, so it is not always profitable (Schemske et al. 1978). Another way would be a late-season growth. However, this strategy is not applicable because spring ephemerals have typical ‘sun-plant’ photosynthetic characteristics, having high light-saturated rate of photosynthesis and light compensation points (Pearcy and Sims 1994, Rothstein and Zak 2001, Horibata et al. 2007). Moreover, a shortened bright period due to early canopy closure effectively restricts carbon assimilation, which greatly reduced subsequent reproductive output due to low photosynthetic production for fruit development and small carbon storage for future reproduction (Lapointe 2001). Spring ephemerals fix the solar energy for survival and reproduction for a short growing period. As a result, the photosynthetic products are not sufficient under unsuitable environment. Nonetheless, small finite carbohydrates must be allocated to two strategies of survival and reproduction. Thus, the correlation between reproduction and storage within plants becomes increasingly negative. Available empirical work indicates that allocation to reproduction vs. storage can be inversely related (Sohn and Policansky 1977). Moreover, the allocation of resources to current reproduction at the expense of survival and future reproduction possibly reflects the selective effects of unpredictable availability of pollinators for this spring ephemeral (Lubbers and Lechowicz 1989).
On the other hand, factors affecting reproduction of plants are very diverse. Physical factors like day length, air temperature, soil water potential, and minerals are important to annual plants’ reproduction. However, not only physical factors but also biological factors like age or size are important to perennial plants (Klinkhamer et al. 1992). Especially, plant size is very important for reproduction (Wesselingh et al. 1997). The reproductive potential depends upon the number of pollinated flowers, the number of fertilized ovules, fruit/seed predation, weather conditions, and the ability of the maternal parent to provide the necessary resources for development (Stephenson 1984). Considering the finite photosynthetic products and two strategies mentioned above, reproduction is often a lethal or semi-lethal activity. Many flowers and fruits are green, and a fraction of the energy and carbon might be obtained by direct photosynthesis within these structures (Biscoe et al. 1975, Bazzaz et al. 1979). In most plants, it is difficult to estimate the energy needed for survival separately from reproduction. First, photosynthetic products can be transferred in diverse organs or directions. Second, the reproductive period and whole growing season are largely overlapping. Third, allocation patterns of photosynthetic products between current reproduction and storage for next season should be a crucial lifehistory strategy (Lubbers and Lechowicz 1989, McKenna and Houle 2000).
Spring ephemerals are a common element of the herb layer and have a short life cycle. Thus, spring ephemerals are worthy of study at a population and community level. In Korea, there were reports on
The aim of the present study was to examine the seed production in a natural
Study area, study period and plants studied were as previously reported (Min 2014). When all petals (corolla) fell, fruit was wrapped in nylon mesh to prevent loss of the seed. When half of the leaves was changed to yellow in late growing season (15-25 May), the shoots of all plants surveyed (including receptacle and peduncle) were harvested. Except for seeds, the shoot was oven-dried at 85℃ for 48 h and weighed. Seeds were divided into two groups according to their length and then counted. Seeds over 3 mm and globular were faithful, and those under 3 mm and flattened were insufficient. However, the insufficient seeds were mostly below 2 mm in length and the size difference between the two groups was clear. To check the biomass of its shoot and root, 94 plants were excavated on May 15, 2009. The sample was classified into two groups of flowering and non-flowering plants regardless of number of flowers. The samples were then divided into shoot and root. All samples were oven-dried at 85℃ for 48 h and weighed. Over-flower (i.e., flower = faithful seed) and under-flower product plants were classified as follows: in over-flower product plant, the plant’s shoot biomass was smaller than
>
Plant size and seed production
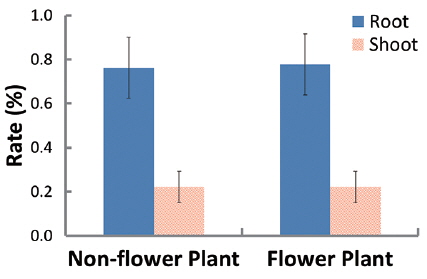
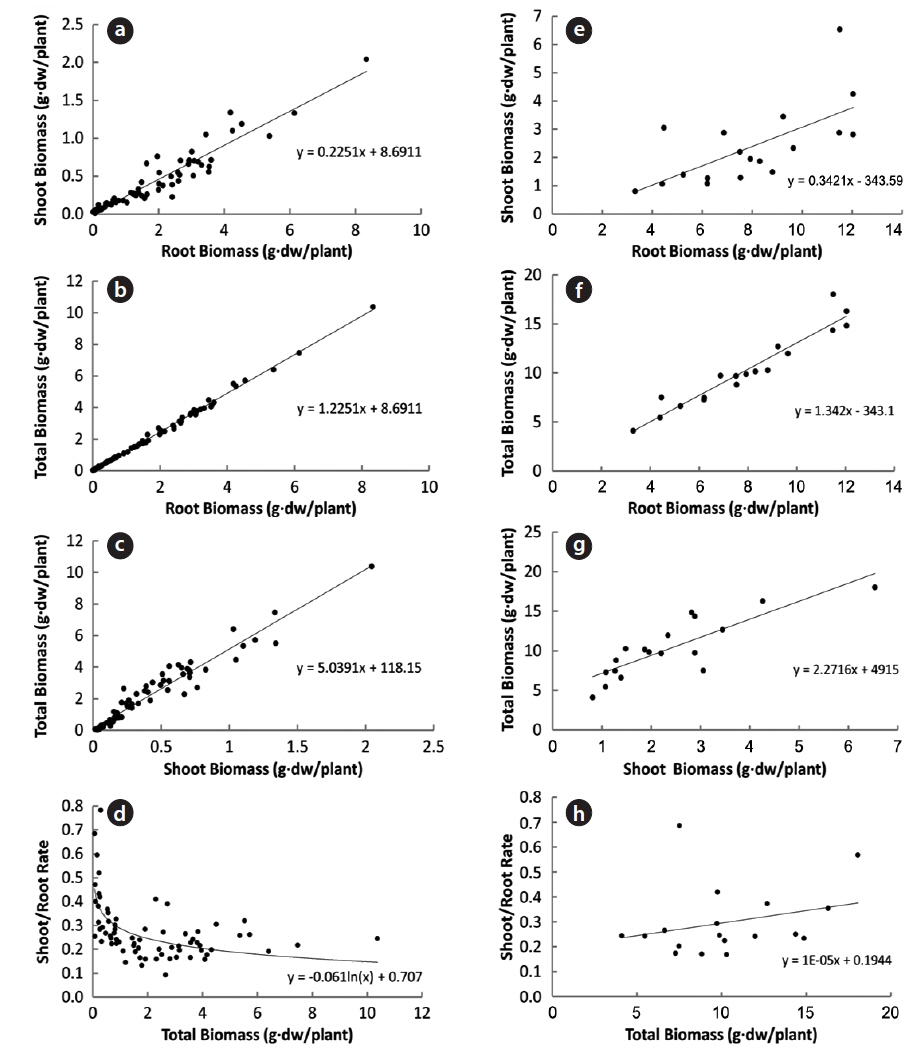
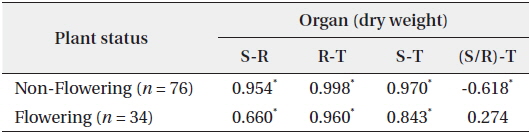
In 94 plants, the biomasses of shoot and root were in the range of 0.014–6.538 g and 0.005–12.038 g, respectively. Thus, the range of sizes was broader in the latter than in the former. The rates of shoot and root were 22% and 78% in non-flowering plants, and 24% and 76% in flowering ones, respectively (Fig. 1). Thus, biomass was larger in the root than in the shoot, regardless of flower production. The biomass of the root was closely related to that of the shoot or total plant (Fig. 2). Correlation coefficients (CC) between the two biomass elements of the shoot, root, and total plant were over 0.6 and significant at the 1% level, except for the CC between shoot/root rate and total plant (Table 1). CCs were higher in the biomass elements of non-flowering plants than in those of flowering ones. CC between root and total plant biomasses of nonflowering plants was the highest while CC between shoot and root biomasses in flowering plants was relatively low. However, in non-flowering plant, shoot/root rate was reversely proportionate to the total biomass with the regression line between these two elements being a curve rather than a line. Three conclusions could be drawn from these results. First,
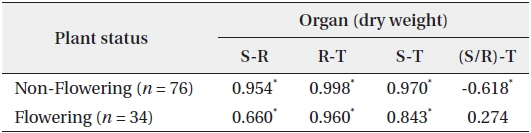
Correlation coefficients between the two biomass factors of the shoot (S), the root (R), the shoot/the root rate (S/R) and the total plant (T) of Adonis multiflora
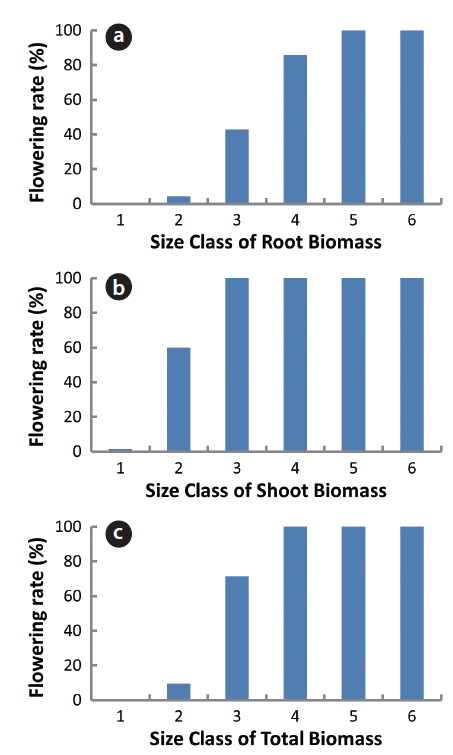
Ninety-four plants were also divided into 6 classes based on shoot, root and total biomasses and the flowering rate of each class was calculated (Fig. 3). In the 2.0-4.0 g root biomass, flowering rate was 5% while it was 100% in the root biomass over 8 g. In the shoot biomass below 1 g, the flowering rate was under 1%; in 1.0-2.0 g of the shoot biomass, the flowering rate was 60%; and over 2 g of the shoot biomass, it was 100%. In the 3-6 g of total plant biomass, the flowering rate was 10%; in 6-9 g, 71%; and over 12 g, 100%. Thus, the flowering rates increased with the increase in all biomasses. However, the increasing trend was gentle with root biomass, but it was steep with shoot or total biomass. Thus, the shoot biomass was the best predictor of flower-producing ability of a plant. A minimum plant size is required before sexual reproduction occurs (Thompson et al. 1991, Schmid and Weiner 1993). Thus, our results in
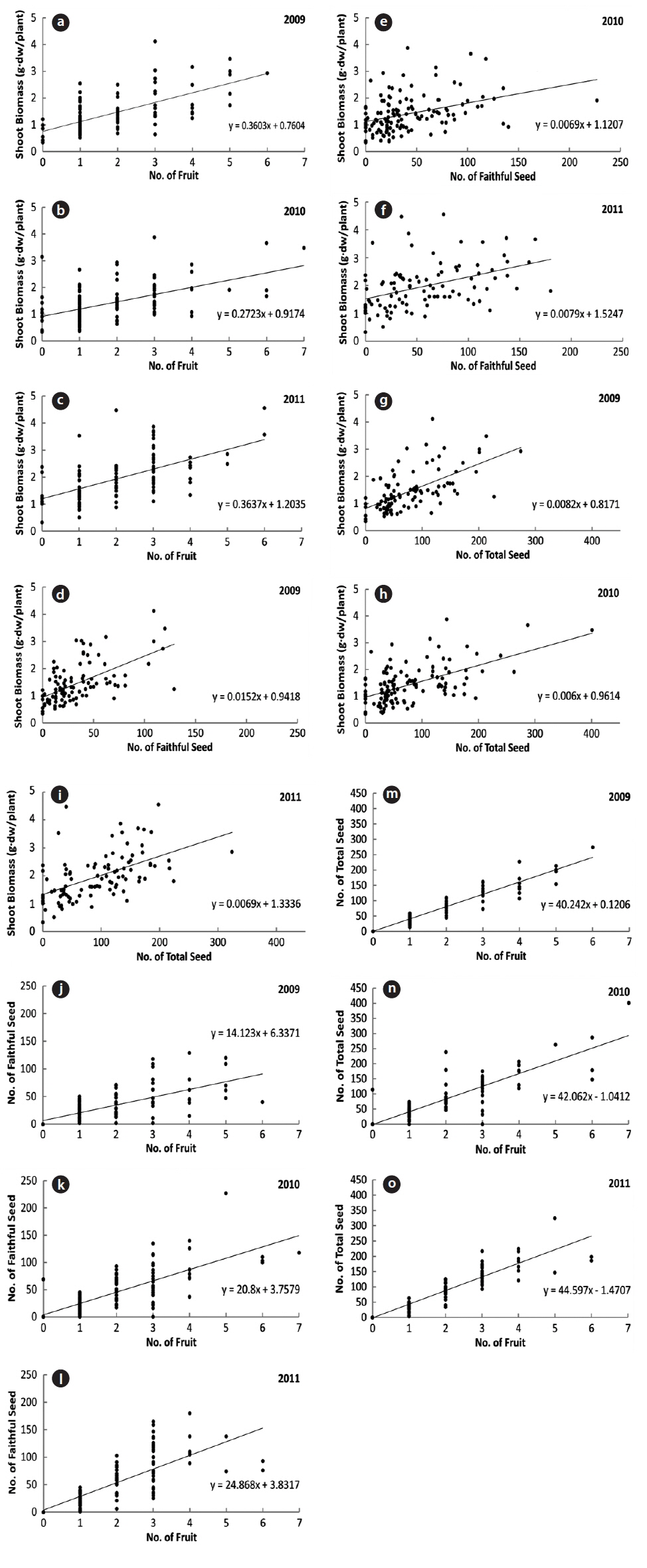
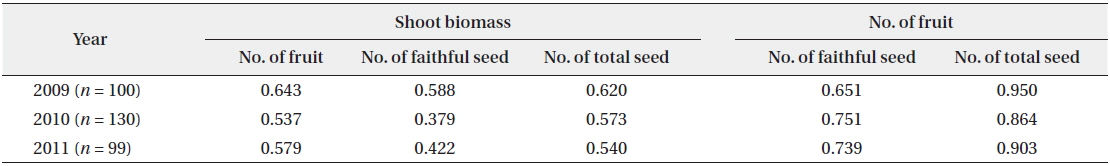
During the three years, the number of fruits, faithful seeds, and total seeds were proportionate to the shoot biomass (Fig. 4). The CCs were significant at the 1% level for all three years (Table 2). Thus, the relationship between the biomass elements and reproductive factors were highly positive. However, considering faithful seeds and other reproductive elements separately, the shoot biomass and number of fruits were more closely related to the number of total seeds than that of faithful ones. Thus, shoot size, number of flowers, and total seeds appear to be determined by the same factors, and the faithful seeds might be analyzed by other methods. The former was mostly fixed before germination and might be affected by root biomass, genetic factors, or embryological properties. Shoot embryos of spring ephemerals are completed in the previous year’s autumn and the maximum number of ovaries in a fruit is genetically fixed (Lapointe and Lerat 2006). The shoot growth in early season was affected by the energy stored in root. However, the faithful seeds were provided the energy from photosynthetic organs during the current growing season. Thus, the number of faithful seeds seems to be influenced by photosynthetic products during the fruiting period. A strong positive correlation between plant size and reproductive output were revealed by many authors (Klinkhamer et al. 1992, Schmid and Weiner 1993, Obeso 2002), but the relationship between plant size and reproductive effort has received little attention (Verburg et al. 1996).
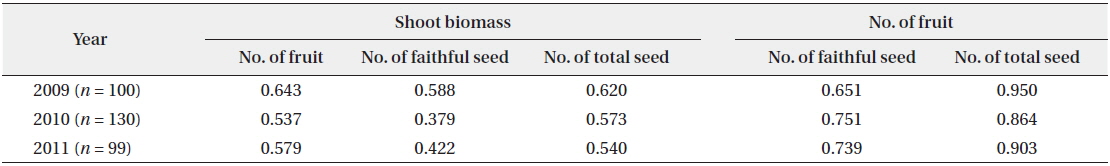
Correlation coefficients between the two factors of number of seed, number of fruit and shoot biomass
>
Seed production and other properties
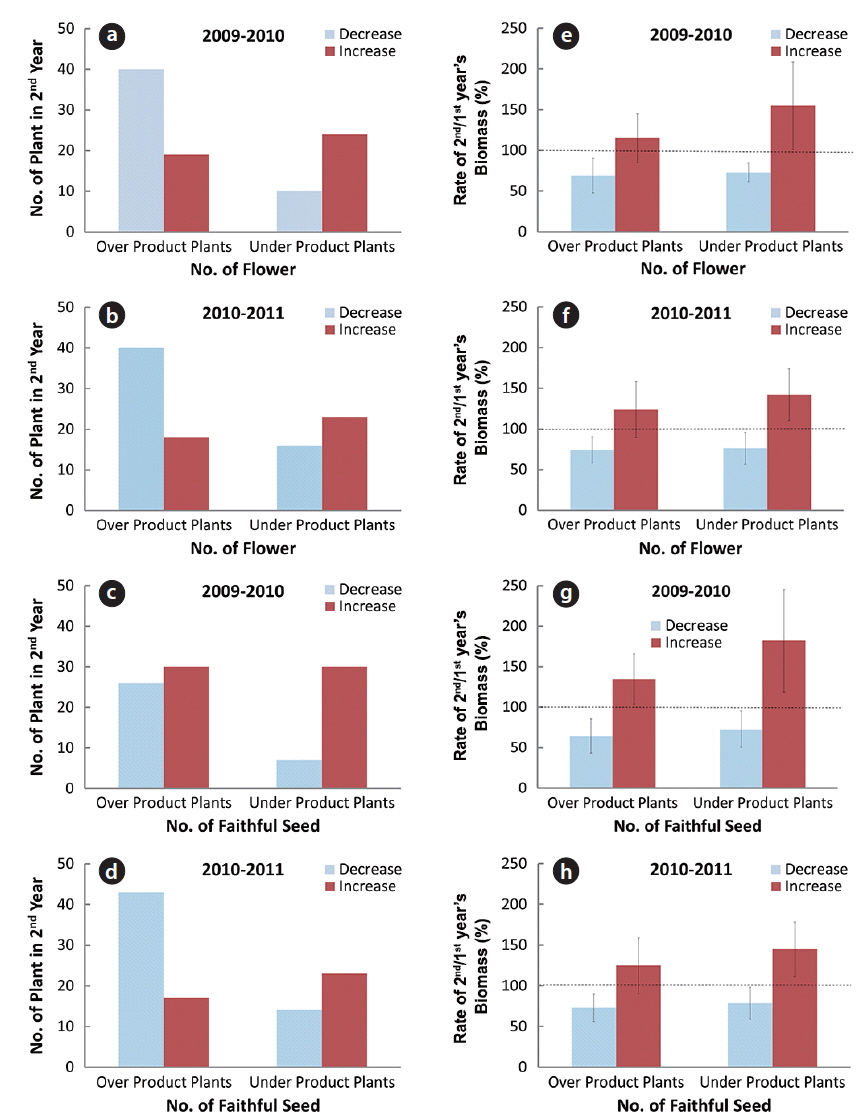
In the flowering plants for two consecutive years, the relative shoot biomass changes between over-flower (faithful seed) product plant and under-flower (faithful seed) one were prominent. The over-flower product plant in one year showed mostly small shoot biomass in the next year and
Based on these results,
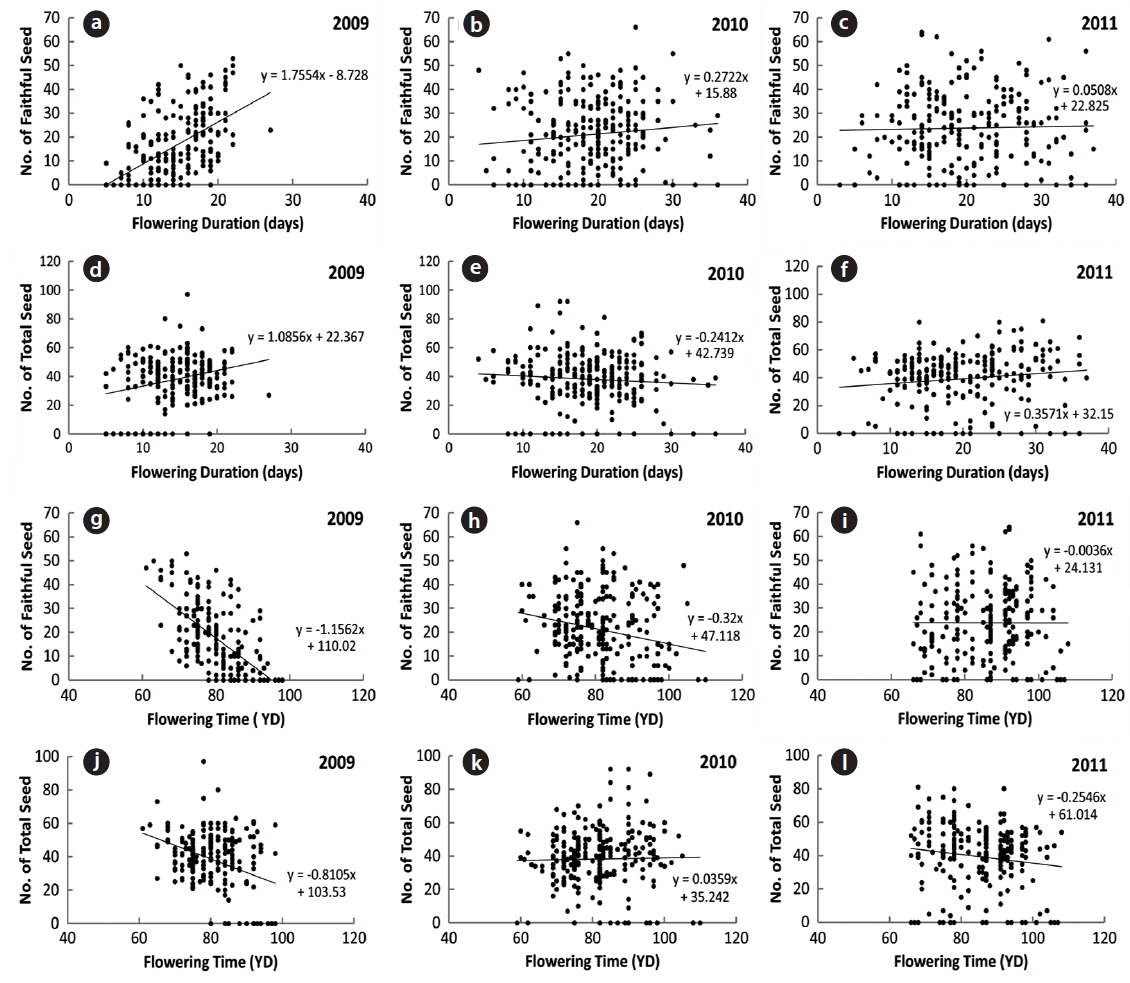
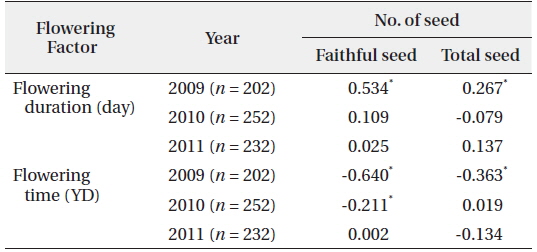
The number of seeds (faithful and total) produced was related to flowering duration in 2009 (Fig. 6). The longer the flowering duration lasted, the more seeds were produced. CC between the number of seeds and flowering duration was significant at the 1% level. However, there was no significant relationship between flowering duration and seed production in 2010 and 2011 (Table 3). Thus, the number of seeds produced was only partly related to the flowering duration. On the other hand, the flowering time affected the number of seeds (faithful and total) in 2009 with CCs between the two factors significant at the 1% level. The earlier flowering time started, the more seeds were produced. However, the relationship between the flowering time and the number of seeds was not evident in 2010 and 2011. Thus, as for flowering duration, the relationship between the flowering times and the number of seeds was not clear. As previously reported, low air temperatures below 0℃ lasted for several days in mid-March of 2010 and 2011, but not in 2009. These low temperatures appear to severely affect the time and duration of flowering. Typically, the natural flowering time maximizes the reproductive output and long flowering duration is favorable to fruiting (Shitaka and Hirose 1998). In spring ephemerals, the earlier flowering time started, the longer flowering duration lasted. However, in early-flowering populations, the low activity of pollinators may result in a quantitative pollen limitation due to low visit frequency under cool conditions, so that flowering in early-season is not always advantageous (Kudo 1993). Flowers with longer duration could receive more energy than those with the short duration ones. Thus, the flowering duration is important in the seed production of spring ephemerals, whose life cycle is short (McKenna and Houle 2000, Rothstein and Zak 2001).
[Table 3.] Correlation coefficients between the number of seed and the flowering factors
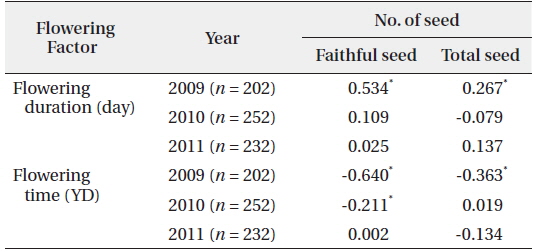
Correlation coefficients between the number of seed and the flowering factors
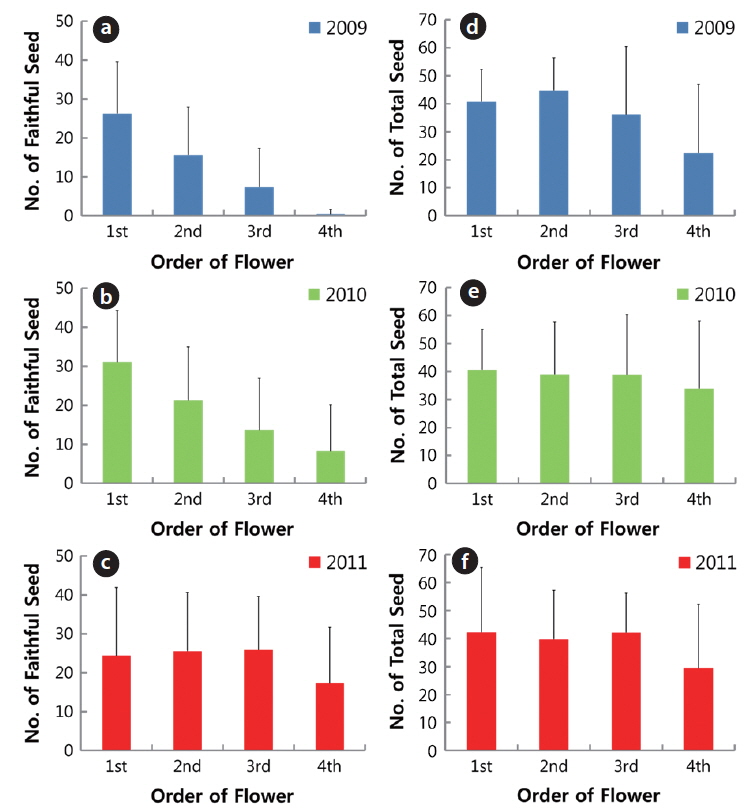
The number of faithful seeds was closely related to inflorescence (the order of flower in a plant), except in 2011 (Fig. 7). The numbers of faithful seeds from first to fourth flower were 26.2 ± 13.4, 15.6 ± 12.3, 7.3 ± 10.0, and 0.4 ± 1.3, respectively, in 2009; and 31.1 ± 13.2, 21.3 ± 13.7, 13.7 ± 13.3 and 8.2 ± 11.9, respectively, in 2010. The differences in seed number between the two consecutive inflorescences from first to fourth flower were significant at the 1% level. However, the total seeds were similar in the first through fourth flowers and the differences in the inflorescence were not significant at the 1% level, except for the result in 2009. Based on this, the number of ovule per flower of