The red algal genus Callophyllis was established by Kützing (1843) for Callophyllis variegata (Bory de SaintVincent) Kützing from Chile and C. laciniata (Hudson) Kützing form Europe (Norris 1957), and it is distributed in the cold and temperate regions of both hemispheres (Arakaki et al. 2011). The genus Callophyllis is distinguished from other kallymeniacean genera by its medullary structure that is composed of large and conspicuous isodiametric cells, and the female reproductive structure that has no separate auxiliary cell systems (Norris 1957, Womersley and Norris 1971). At first, species delimitation was carried out by observing the simple vegetative features of Callophyllis species distributed in the west coast of North America (Setchell 1923), but the identification of species was conducted using the reproductive structures such as the position and size of cystocarps, number and shape of ostioles, and presence or absence of tetrasporangia sori (Dawson 1954, Norris 1957, Abbott and Norris 1966). Abbott and Norris (1966) indicated that identification based on only vegetative features has led to errors when determining species diversity, because considerable variation occurs in some Callophyllis species from the Pacific coast of North America.
The advancement of the molecular method for the taxonomic study of red algae has allowed us to overcome the limit of morphological observations, to recognize species diversity with the discovery of new species, and to infer the phylogenetic relationships among taxa (Saunders 2008, Koh et al. 2013, Schneider et al. 2014). In the genus Callophyllis, Harper and Saunders (2002) carried out molecular analysis using the nuclear LSU rDNA gene marker. Consequently, they confirmed the monophyletic clade of the genus Callophyllis in the family Kallymeniaceae and transferred three Callophyllis species to Euthora and Pugetia: C. cristata to Euthora, C. firma, and C. chilensis to Pugetia (Harper and Saunders 2002). Arakaki et al. (2011) studied the species delimitation of Callophyllis from the central-southern Chile using the plastid rbcL gene and newly reported C. concepcionensis and C. macrostiolata. Clarkston and Saunders (2012) established the new genus, Salishia, by examining the mitochondrial COI-5P gene and other DNA markers. Furthermore, Clarkston and Saunders (2013) resolved the species diversity of Callophyllis that is distributed in the northeastern Pacific coastline, including a new species, C. schneideri, and Klochkova et al. (2013) described Ionia cornu-cervi as a member of Callophyllis using rbcL analysis combined with morphological evidence. Although the species delimitation of the genus Callophyllis from the eastern Pacific of both hemispheres was well-established, based on the morphology and molecular evidence, it remains an unresolved taxonomic group in the western Pacific region.
In the western Pacific region of the northern hemisphere, there are currently eight recorded Callophyllis species (Yoshida 1998), including six species from Korea (Lee and Kang 2001) and three in China (Xia 2004): Callophyllis adhaerens Yamada, C. adnata Okamura, C. crispata Okamura, C. hayamensis Yamada, C. japonica Okamura, C. mageshimensis Tanaka, C. palmata Yamada, and C. rhynchocarpa Ruprecht. C. adhaerens has branches that adhere to each other or other things, which was recognized as a unique characteristics (Yamada 1932). C. adnata is characterized by its softly membranous and horizontally attached by radical discs (Okamura 1932). C. crispata has an erect, palmate or flabellate thallus, 10–20 cm high, and 20–30 mm wide, with a crenulated margin (Okamura 1896). C. crispata and C. palmata have a similar external habit, including the coriaceous substance, but they are distinguished by the positon of the cystocarps (Okamura 1916, 1932, Yamada 1930). C. japonica is distinguished by its pinnately subdichotomous thallus in irregular, growing spinescent protuberances at the margin, and the location of cystocarps (Okamura 1936). The above recorded species, except C. rhyncocarpa, were considered endemic in the northwestern Pacific (Guiry and Guiry 2014).
The species of Callophyllis mostly grow in the subtidal zone, but specimens have hardly been collected from this area, because of the difficulty of approaching deep water. Therefore, we have continuously surveyed subtidal habitats around Jeju Island, Korea, to discover cryptic species of the red algae. The aims of this study are to clarify the species delimitations of Callophyllis, based on its morphology and molecular analyses of plastid rbcL and mitochondrial COI-5P, to discover cryptic species diversity in the subtidal zone of the Korean coast by applying molecular tools, and to understand the phylogenetic relationships of Callophyllis species that occur in the northwestern Pacific.
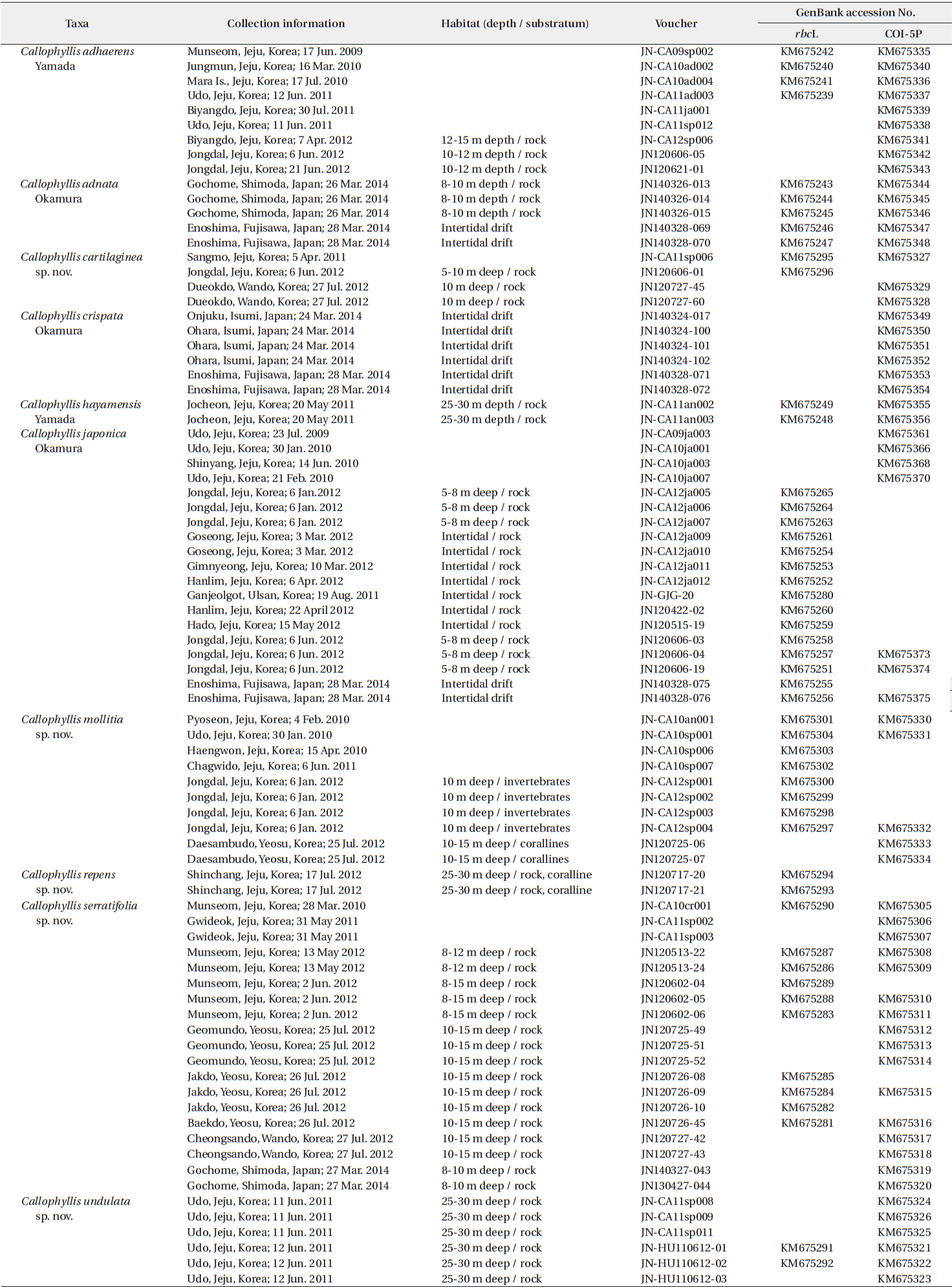
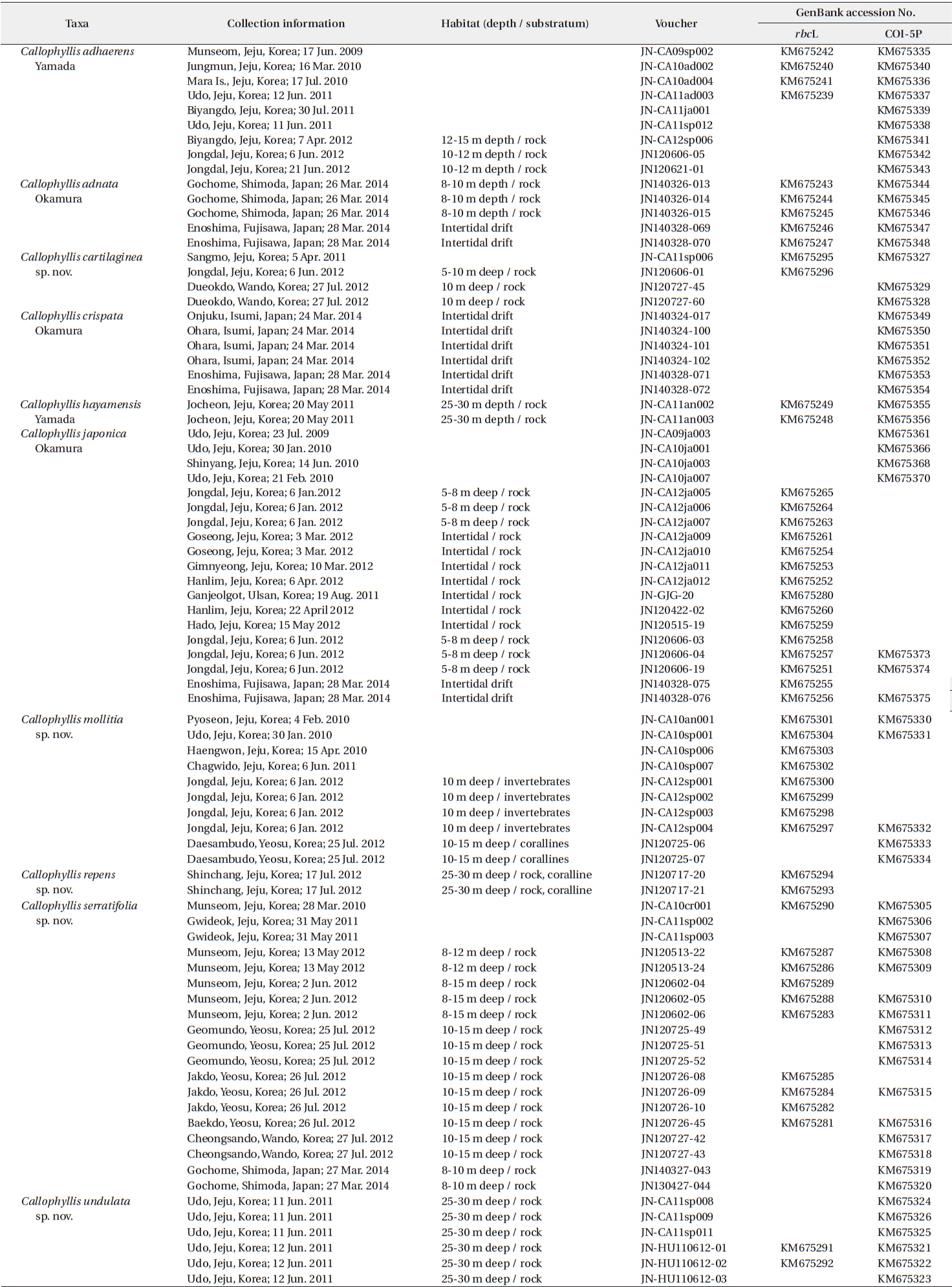
The Callophyllis specimens examined in the present study are listed in the Table 1. The collections of samples were conducted in the intertidal and subtidal zones by SCUBA diving in Korea and Japan, including the type locality, Enoshima. Samples were transported to the laboratory, and photographed by a D-80 camera (Nikon, Tokyo, Japan). The samples for DNA extraction were detached from the thallus to make silica-gel-dried tissue samples. The voucher specimens were made into a pressed one on the herbarium paper and deposited in the herbarium of the Jeju National University (JNUB), Jeju, and in the National Institute of Biological Resources (NIBR), Incheon, Korea. Samples for anatomical study were preserved in 4–5% formalin in seawater. After describing the external morphology by using a dissecting microscope SZ61 (Olympus, Tokyo, Japan), sections were done by hand using a razor or a freezing microtome (NK-101-II; Nippon Optical Works Co. Ltd., Tokyo, Japan). In addition, specimens for squash preparations were soaked for 1–2 h in a 5% solution of 5 N KOH, then rinsed in distilled water for 1 h (Schneider et al. 2014). Sections and squash preparations were stained with 1% acidified aniline blue and mounted in 30% corn syrup. The Wittmann’s aceto-ironhematoxylin chloral hydrate staining method (Wittmann 1965) was used for precise observations of the carpogonial branches with 50% Hoyer’s mounting medium (Lin et al. 2012). Photomicrographs were taken using a QImaging 1394 camera (QImaging, Surrey, BC, Canada) attached to a BX50 microscope (Olympus, Tokyo, Japan).
DNA was extracted using DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s protocol. Extracted DNA was processed to the polymerase chain reaction (PCR) to amplify rbcL and COI-5P genes. The primer combinations used to amplify each gene region were rbcL_F145-rbcL_R898 and rbcL_F762-rbcL_R1442 (Kim et al. 2012), and GazF1-GazR4 in COI-5P (Saunders 2008). PCR amplification was conducted in a total volume of 20 μL with AccuPower PCR PreMix (Bioneer, Daejeon, Korea) using Peltier Thermal Cycler, PTC-100 (BIO-RAD, CA, USA). Each PCR profile of rbcL and COI-5P was followed by Saunders and Moore (2013). PCR products were purified using an AccuPrep Purification Kit (Bioneer), and then all sequencing was outsourced to Macrogen (Seoul, Korea). Both electropherogram outputs from each sample were edited using Chromas ver. 1.45 (Technelysium Pty Ltd., Helensvale, QLD, Australia). The total rbcL and COI-5P sequences were organized using the multiple-sequence editing program BioEdit (Hall 1999) and aligned visually.
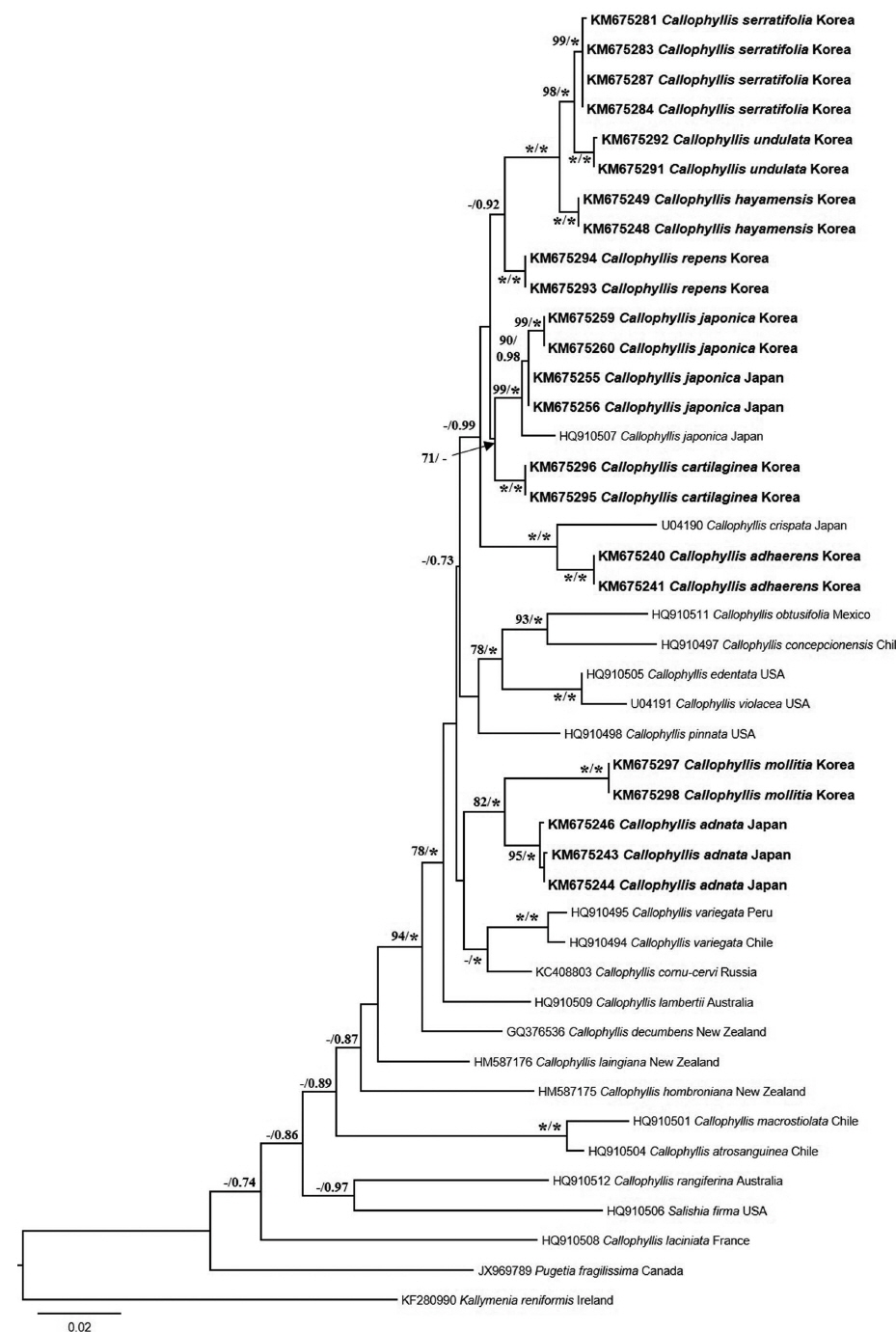
A neighbor-joining analysis of COI-5P alignment with distance corrected under a Kimura 2-parameter model was completed using MEGA ver. 5.10 (Tamura et al. 2011). For rbcL phylogeny of the genus Callophyllis, our specimens were analyzed with sixteen Callophyllis species from GenBank including the generitype Callophyllis variegata (HQ910494), and three outgroups: Pugetia fragilissima (JX969789), Salishia frima (HQ910506), and Kallymenia reniformis (KF280990). Maximum likelihood (ML) analyses were performed using RAxML software (Stamatakis 2006). We used the “-f a” option for rapid bootstrap analysis, and best likelihood tree searching using “-# 1000” and 200 independent tree inferences with the “number of run” option, with default-I and an automatically optimized subtree pruning and regrafting (SPR) rearrangement to identify the best tree. To generate bootstrap values for the phylogeny, the statistical support for each branch was obtained from 1,000 replications, using the same substitution model and RAxML program settings. In addition, we performed Bayesian phylogenetic inferences (BI) using MrBayes ver. 3.1.2 (Ronquist and Huelsenbeck 2003) with the Markov Chain Monte Carlo (MCMC) to get the Bayesian posterior probablities.
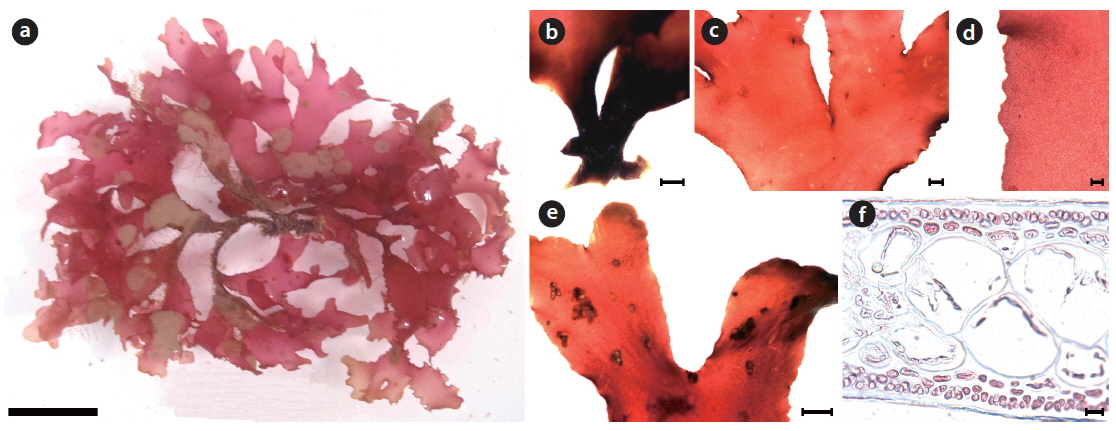
Callophyllis cartilaginea H. W. Lee & M. S. Kim sp. nov. (Fig. 3)
Description: Thallus cartilaginous, erect, narrowly flabellate, dark- to brown-red in color, di- or trichotomously branched in a bushy clump, stipitate in slightly compressed, 5-7 cm long, 1.1-1.7 mm wide in the middle, attached to the substratum by a sub-discoidal holdfast; apex acute; margin usually smooth with spinescent protuberances.
Holotype: JN120606-01 (Fig. 3a), vegetative plant, collected at 5–10 m in depth of the subtidal zone on 06 June 2012 and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Type locality: Jongdal, Jeju, Korea (33°29′34.40″ N, 126°55′16.23″ E).
Etymology: The specific epithet (cartilaginea) was chosen to represent this species, as it has a cartilaginous thallus.
Distribution: Korea (this study).
Selected specimens: JN120606-01-2 (Jongdal, 06 June 2012, vegetative); 120727-45, 60 (Dueokdo, 27 July 2012, vegetative); NIBRAL0000143264, 140423-s01-3 (Jongdal, 23 April 2014, vegetative).
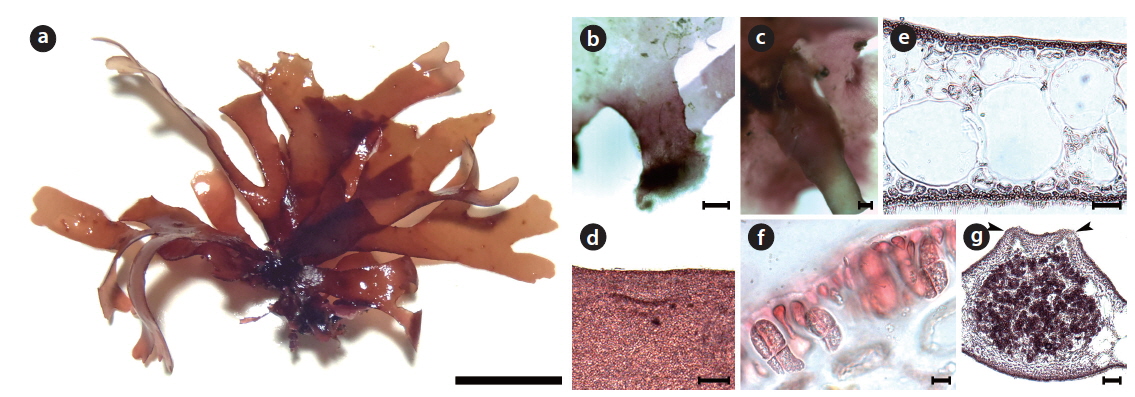
Morphology: Plants are erect, flabellate, cartilaginous, and dark-red in color (Fig. 3a). Thallus has a short subcylindrical stipe arising from a discoidal holdfast, which gets more compressed (Fig. 3b). The thallus is di- or trichotomously branched (Fig. 3c), and is irregularly subdichotomously branched at the upper portion (Fig. 3d). The thallus is 5–7 cm long, 310–358 μm thick, and 1.1–1.7 mm wide, but 2.2–3.4 mm wide at the branching point. The thallus margin is almost smooth, and often small spinescent protuberances laterally (Fig. 3c and 3d). The apex is acute (Fig. 3d). The medulla is made of a two- or threecelled layer: Two large isodiametric cells, 112–146 μm × 104–160 μm in size, and one small oblong cell, 49–61 μm × 58–72 μm in size (Fig. 3e). Outer cortical cells are beadlike and 4.3–5.6 μm × 4.5–5.4 μm in size, whereas inner cells are oblong and 4.1–5.7 μm × 6.4–8.5 μm in size.
Callophyllis mollitia H. W. Lee & M. S. Kim sp. nov. (Fig. 4)
Description: Thallus membranous, tender, flattened, palmate, bright brown-red in color, sub-dichotomously branched, shortly stipitate, 3–5 cm long, 4.3–5.1 mm wide in the middle, attached to the substratum by a discoidal holdfast with several minute radical discs arising from the thallus; apex roundish, margin smooth, cystocarps hemispherical scattered over the entire blades with 2–3 onesided ostioles; tetrasporangia cruciately divided, scattered irregularly in the cortex.
Holotype: JN120725-07 (Fig. 4a), female gametophyte, collected at 12–15 m in depth of the subtidal zone on 25 July 2012 and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotype: NIBR (NIBRAL0000143265, vegetative) and JNUB (JN120725-08, ♀).
Type locality: Daesambudo, Yeosu, Korea (34°03′07.17″ N, 127°22′56.58″ E).
Etymology: The specific epithet (mollitia) was chosen to represent the tender thallus of this species.
Distribution: Korea (this study).
Selected specimens: JN12sp001-4 (Jongdal, 06 January 2012, ⊕ ); NIBRAL0000143265 (Daesambudo, 25 July 2012, vegetative), 120725-07-8 (Daesambudo, 25 July 2012, ♀); 120130-16-18 (Munseom, 30 January 2013, vegetative).
Morphology: Plant is flattened, palmate, tender, membranous, and bright brown-red in color (Fig. 4a). Its thallus is branched irregularly 2–3 times (Fig. 4a). The thallus has a very short stipe arising from a radical disc (Fig. 4b), and is attached by several minute radical discs borne laterally or ventrally at the basal portion (Fig. 4c). The thallus is 3–5 cm long, 279.8–315.7 μm thick, 1.9–2.9 mm wide at the basal, 4.3–5.1 mm wide at the middle, and 2.3–2.9 mm at the upper portion. The thallus margin is smooth all over (Fig. 4d). The apex is roundish or bluntly dissected. The medulla is made up of irregular isodiametric or subisodiametric cells (Fig. 4e), the smaller cells are 75–83 μm × 80–110 μm in size, and the larger cells are 141–204 μm × 152–198 μm. The cortex is made up of bead-like cells, 5.3–5.9 μm × 4.9–6.1 μm in size (Fig. 4e). Tetrasporangia are oblong and cruciately divided, and they are located in the cortex, 31.1–34.5 μm × 13.0–15.6 μm in size (Fig. 4f). Cystocarps are scattered over the entirety of the blades, more protuberant dorsally than ventrally, and they have several ostioles opened dorsally (Figure 4g). The cystocarps are 738.6–784.6 μm × 795.3–818.7 μm.
Callophyllis repens H. W. Lee & M. S. Kim sp. nov. (Fig. 5)
Description: Thallus creeping, flattened, flabellate, slightly cartilaginous, dark red in color, dichotomously branched, 2–5 cm long, 2.1–2.8 wide in the middle, attached to the substratum by a discoidal holdfast with several minute radical discs; apex is acute; margin minutely crenulated, a few globular lateral protuberances, and adherent laterally to neighboring blade margins.
Holotype: JN120717-20 (Fig. 5a), vegetative plant, collected at 25–30 m in depth of the subtidal zone on 17 July 2012 and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotype: NIBR (NIBR0000143263, vegetative).
Type locality: Shinchang, Jeju, Korea (33°21′00.88″ N, 126°09′51.26″ E).
Etymology: The specific epithet (repens) was chosen to represent the creeping thallus of this species.
Distribution: Korea (this study).
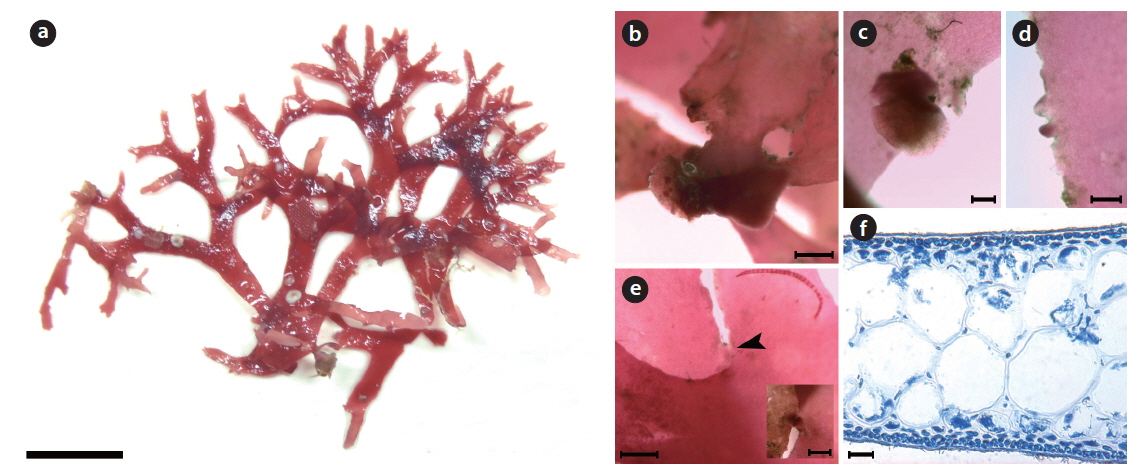
Morphology: Plants are flattened, slightly cartilaginous, and dark-red in color (Fig. 5a). The thallus is dichotomously branched in the same plane (Fig. 5a). The thallus arises from a main discoidal holdfast (Fig. 5b) and bears several minute radical discs at the basal portion (Fig. 5c). The thallus is 2–5 cm long, 200–220 μm thick, and 2.1–2.8 mm wide at the middle portion, getting narrower to the upper portion, 0.9–1.7 mm wide. The apex is acute. The thallus margin is minutely crenulated (Fig.5d). One blade of the thallus is adhered to another blade at the margin by the lateral protuberances of both blades (Fig. 5e). The medulla is made up of irregular isodiametric or sub-isodiametric cells, 61.8–90.1 μm × 58.6–84.9 μm in size (Fig. 5f). The cortex is a one- or two-celled layer, 5.3–6.7 μm × 5.2–6.9 μm in size (Fig. 5f).
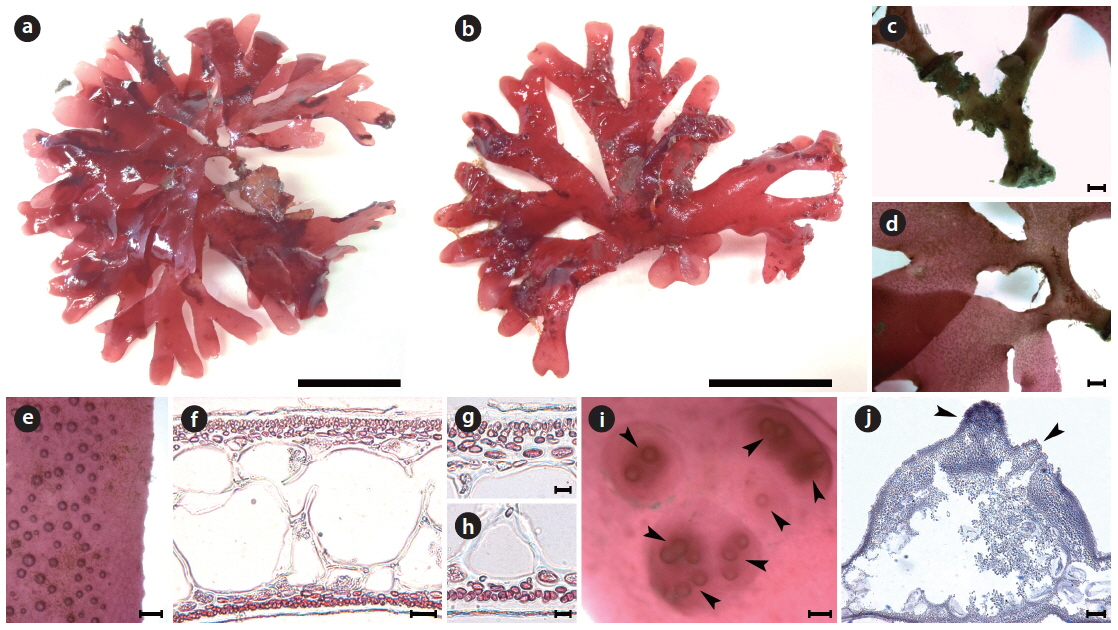
Callophyllis serratifolia H. W. Lee & M. S. Kim sp. nov. (Fig. 6)
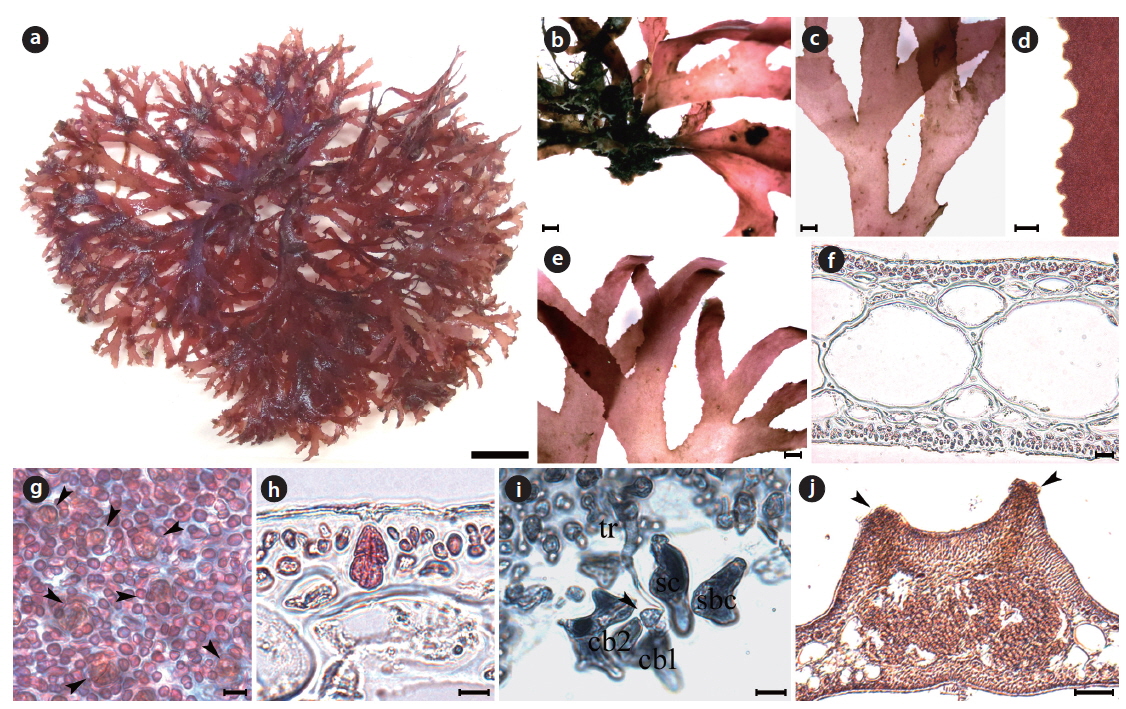
[Fig. 6.] Callophyllis serratifolia H. W. Lee & M. S. Kim sp. nov. (a) Holotype specimen (JN120602-04, vegetative plant, Munseom, 02 June 2012). (b) A discoidal holdfast bearing several fronds. (c) Thallus dichotomously branched. (d) The serration at the margin of frond. (e) Frond having narrowly blunt apex (f ) Cross section of frond showing two celled layer of cortex, and large isodiametric cells of central medulla surrounded by small cells. (g) Surface view showing scattered tetrasporangia (arrowheads). (h) Tetraspoarangia cruciately divided in the cortical layer. (i) Three celled carpogonial branch and carpogonium (arrowhead) borne supporting cell with subsidiary cells. (j) Cross section of cystocarp with two ostioles (arrowheads). Scale bars represent 1 cm (a), 1,000 μm (b, c, e), 100 μm (d, j), 20 μm (f), 10 μm (g-i).
Description: Thallus flattened, flabellate, slightly cartilaginous, dark red in color, dichotomously branched in a bushy clump, shortly stipitate, 5–7 cm long, 2.3–4.9 mm wide in the middle, attached to the substratum by a discoidal holdfast; apex narrowly blunt; margins serrated; procarps consist of a supporting cell, 2–4 subsidiary cells, three-celled carpogonial branch; cystocarps spherical and scattered over the upper blades with 2–6 both-sided ostioles; tetrasporangia cruciately divided and scattered irregularly in the cortex.
Holotype: JN120602-04 (Fig. 6a), vegetative plant, collected at 8–15 m in depth of the subtidal zone on 02 June 2012 and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB)
Isotype: JNUB (JN120602-5, ♀) and NIBR (NIBR0000143261, vegetative).
Type locality: Munseom, Seogwipo, Korea (33°13′40.25″ N, 126°34′01.05″ E).
Etymology: The specific epithet (serratifolia) was chosen to represent the fact that the marginal outline of this species has serrated edges.
Distribution: Korea (Lee 2008) and Japan (this study).
Selected specimens: JN120513-22, 24 (Munseom, 13 May 2012, vegetative); JN120602-04, NIBR0000143261 (Munseom, 02 June 2012, vegetative); JN120602-05 (Munseom, 02 June 2012, ♀); JN120725-49-52 (Geomundo, 28 July 2012, vegetative); JN120727-43 (Dueokdo, 27 July 2012, ⊕); 120727-44 (Dueokdo, 27 July 2012, ♀); JN2013- v0285 (Seopseom, 05 April 2013, ⊕); 130616-25 (Munseom, 16 July 2013, ♀).
Morphology: Plants are flattened, flabellate, slightly cartilaginous, and dark-red in color (Fig. 6a), arising from a short cylindrical stipe. The thallus grows as a single blade or a small clump with several blades from a discodial holdfast (Fig. 6b). The thallus is usually 5–7 cm long, occasionally up to 10 cm long, 2.3–4.9 mm wide at the middle branches, 1.2–2.1 mm wide at the upper part, 320–395 μm thick at the basal, and 193–212 μm thick at the middle. The thallus is dichotomously branched in the same plane (Fig. 6c), and serrated at the margins (Fig. 6d). The apex is narrowly blunt (Fig. 6e). The medullar consists of large sub-isodiametric central cells, 97–102 μm × 101–127 μm in diameter, surrounded by several small cells, 54–71 μm × 55–82 μm in diameter (Fig. 6f). The cortex is usually a two-cell layer consisting of cortical cells, 4.6–6.7 μm × 5.2–7.8 μm in diameter (Fig. 6f). Tetrasporangia are irregularly scattered on the thallus surface (Fig. 6g), and are cruciately divided in the cortical layer, 2.0–2.3 μm × 9.5–10.9 μm in diameter (Fig. 6h). The procarp consists of three-celled carpogonial branches arising from a supporting cell with two subsidiary cells (Fig. 6i). Cystocarps are located on the surface of the upper branches, are 890.4–842.4 μm x 870.6–920.9 μm, and protude toward both sides with both-sided ostioles (Fig. 6j).
Callophyllis undulata H. W. Lee & M. S. Kim sp. nov. (Fig. 7)
Description: Thallus flattened, flabellate, membranous, erect, stipitate, light scarlet to bright red in color, dichotomously branched in a bushy clump, 3–8 cm long, 2.8–9.9 mm wide in the middle, attached to the substratum by a discoidal holdfast; apex roundish; margin pleated.
Holotype: JNHU110612-01 (Fig. 7a), vegetative plant, collected at 25–30 m in depth of the subtidal zone on 12 June 2011 and deposited in the Herbarium of Department of Biology, Jeju National University, Korea (JNUB).
Isotype: JNUB (JNHU110612-2, vegetative) and NIBR (NIBR0000143262, vegetative).
Type locality: Udo Channel, Jeju, Korea (33°29′55.19″ N, 126°56′02.53″ E).
Etymology: The specific epithet (undulata) was chosen to represent the pleated edge of the marginal outline of this species.
Distribution: Korea (this study).
Selected specimens: JNHU110612-01 & 03, NIBR0000143262 (Udo Channel, 12 June 2011, vegetative).
Morphology: Plants are erect, flabellate, scarlet to bright-red in color (Fig 7a), and have a cylindrical stipe that arises from a discoidal holdfast (Fig. 7b). The thallus is 3–8 cm long, 2.8–9.9 mm wide, and 99.1–190.5 μm thick. The thallus is dichotomously branched (Fig. 7c), pleated, and bluntly serrated at the margin (Fig. 7d). The apex is roundish (Fig. 7e). The medulla is almost entirely a twocelled layer of large sub-diametric cells, 42.2–85.4 μm × 39.6–86.6 μm in size (Fig. 7f). The cortex is one or two layered: the outer cortical cells are bead-like, 3.5–5.2 μm × 3.3–5.0 μm in size, and the inner cortical cells are oblong, 2.5–5.2 μm × 6.4–8.6 μm in size (Fig. 7f).
Callophyllis hayamensis Yamada 1941 (Fig. 8)
[Fig. 8.] Callophyllis hayamensis Yamada. (a) JN2013-v0454, vegetative plant collected from Sasu-dong on 07 July 2013, showing flattened membranous thallus. (b) JN2013-v0495, female gametophyte collected from Sasu-dong on 12 July 2013. (c) Cylindrical and dichotomous branched stipe arising from a discoidal holdfast. (d) Thallus dichotomously branched at the basal part. (e) Slightly crenulation at the margin of frond. (f ) Cross section of the frond showing medulla and cortex. (g) Cross section of the dorsal cortex. (h) Cross section of the ventral cortex. (i) Cystocarp borne at the upper part of thallus having ostioles (arrowheads). (j) Cross section view of cystocarp protuberated dorsally with two ostioles (arrowheads). Scale bars represent 2 cm (a, b), 1,000 μm (c, d), 200 μm (e, i), 25 μm (f), 10 μm (g, h), 100 μm (j).
Holotype: TNS (Yoshida 1998).
Type locality: Hayama, Kanagawa, Japan.
Distribution: Korea (this study) and Japan (Yoshida 1998).
Selected specimens: JN2013-v0454 (Sasu-dong, 07 July 2013, ♀); JN2013-v0457-460 (Guideok 1-ri, 10 July 2013, vegetative); JN2013-s0222-4, JN2013-v0494, NIBR0000143266 (Sasu-dong, 12 July 2013, ♀)
Morphology: Plant is flattened, flabellate, scarlet to dark-red in color, 5–8 cm long, and 190–206 μm thick (Fig. 8a and 8b). The thallus has a cylindrical stipe arising from a discoidal holdfast and is 3.9–7.3 mm long (Fig. 8c). The thallus is dichotomously branched 2–4 times, and broadens upwardly (Fig. 8d). The width of the thallus is ranged from 6.4–8.1 mm in the middle to 3.5–4.3 mm in the upper. The margin is slightly crenulated (Fig. 8e). The apex of the thallus is obtuse and roundish. The medulla consists of two isodiametric celled layer, which are 62–114 μm × 46–114 μm in size (Fig. 8f). The outer cortex is dorsiventral, consisting of a two- or three-celled layer in the dorsal cortex and a one-celled layer in the ventral cortex (Fig. 8g and 8h). Cystocarps are scattered over the upper blades (Fig. 8i) and protude more dorsally with several one-sided ostioles (Fig. 8j).
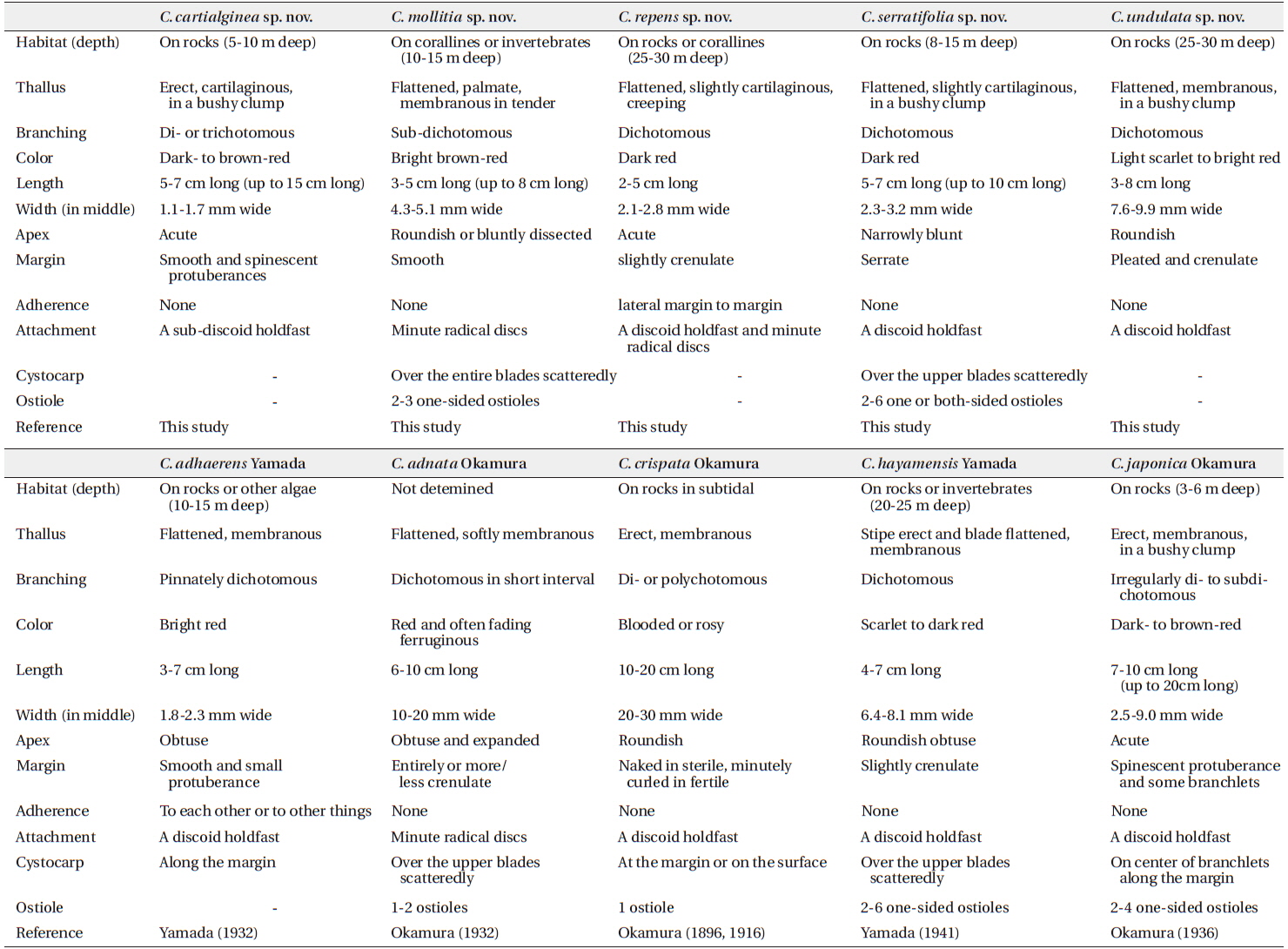
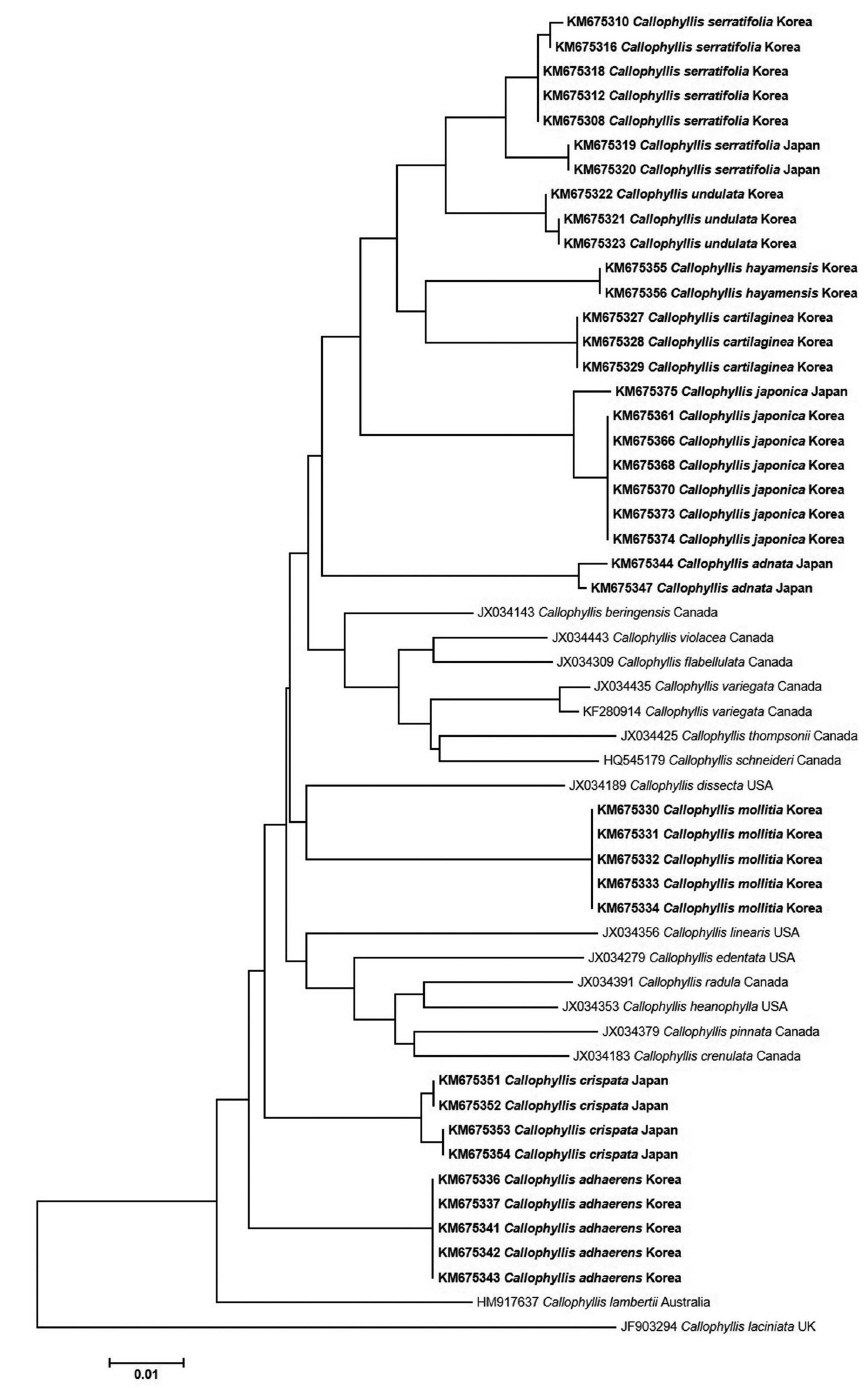
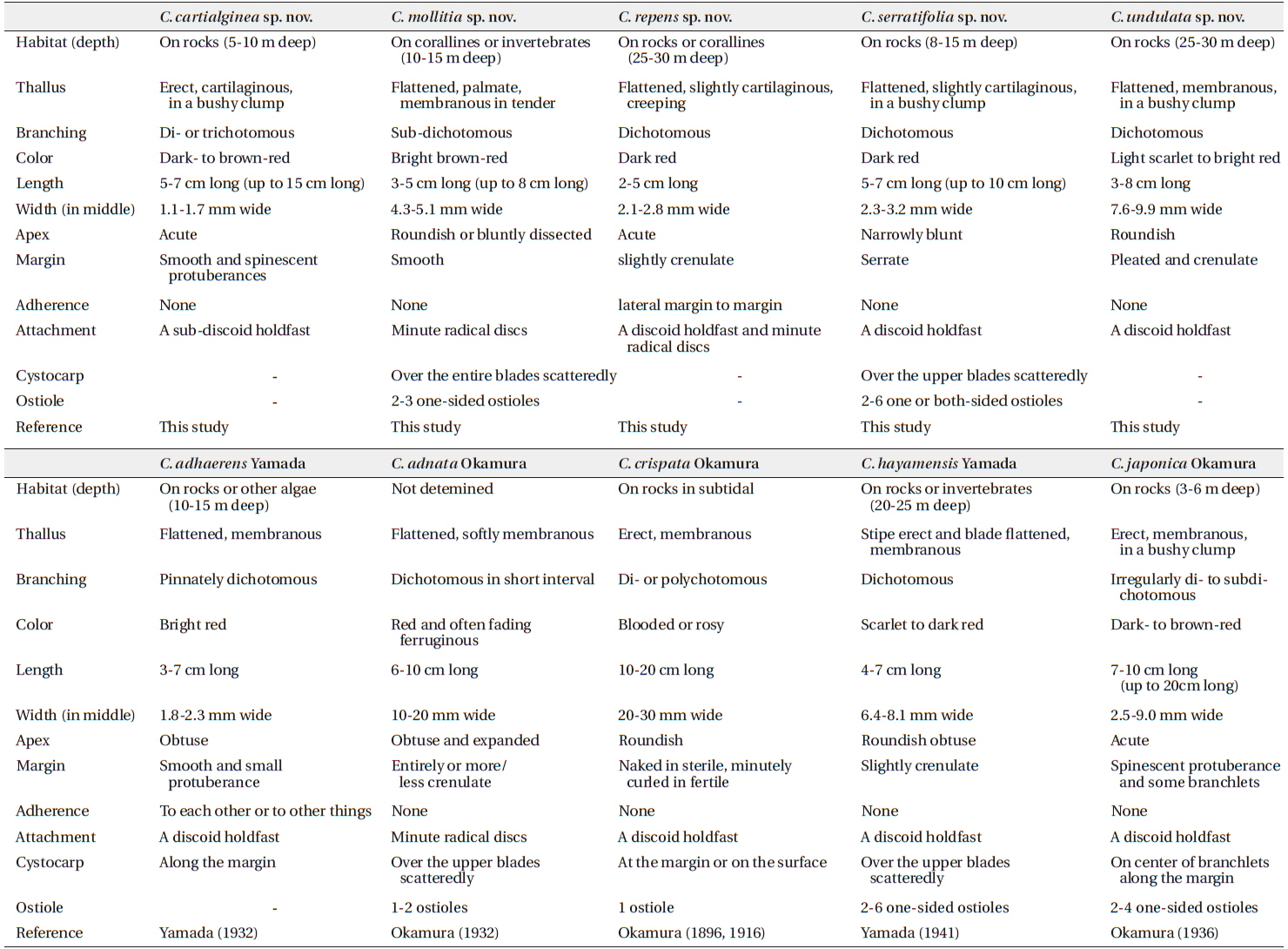
In the present study, we recognized a total of ten Callophyllis species, including five new cryptic species and one unrecorded species from Korea based on the morphology with molecular analyses of the plastid rbcL and mitochondrial COI-5P. We proved five Callophyllis species that have been previously recorded in the northwestern Pacific (Yoshida 1998, Xia 2004, Lee and Kang 2001): Callophyllis adhaerens Yamada, C. adnata Okamura, C. crispata Okamura, C. hayamensis Yamada, and C. japonica Okamura. Two species, C. adnata and C. crispata, were only collected from Japan in this study, and the distribution of C. hayamensis was expanded to Korea. In particular, the diversity of Callophyllis from the northwestern Pacific was increased by discovering five new cryptic species: C. cartilaginea sp. nov., C. mollitia sp. nov., C. repens sp. nov., C. serratifolia sp. nov., and C. undulata sp. nov.
To delimit the species boundaries of the genus Callophyllis, morphological features have been compared such as thallus type and size, branching pattern, marginal type, position and size of cystocarps, number and shape of ostioles, and presence of tetrasporangia sori (Sectchell 1923, Dawson 1954, Norris 1957, Abbott and Norris 1966, Womersley and Norris 1971). We compared ten Callophyllis species on the basis of the combination of the above morphological features, and consequently defined five new species in this study that could be discriminated from other species (Table 2). C. cartilaginea sp. nov. can be mistaken as C. japonica, but two species differ in thallus type and the shape of stipe: the former species has a cartilaginous thallus with a short compressed stipe, and the latter has a membranous thallus with apparent cylindrical stipe and spinescent protuberances at the lateral margin (Okamura 1936). C. mollitia sp. nov. resembles C. adnata, as they both have membranous blades in tender and are attached by several radical discs (Table 2), but the thallus of C. adnata is more than twice as wide, with a red and often fading ferruginous color and slightly crenulated margins (Okamura 1932). C. repens sp. nov. is similar to C. mageshimensis in its creeping habit, attached to the substratum with several radical discs, although C. mageshimensis has no adherent branches and an apparently acute apex (Tanaka 1963). C. serratifolia sp. nov. is characterized by its narrowly blunt apex, regularly dichotomous branching pattern, and serrated margin without pleat and both-sided cystocarp ostioles, and it is clearly distinguished from any other species distributed in the northwestern Pacific (Table 2). C. undulata sp. nov. is similar to C. hayamensis on the thallus type and width and apex shape, but C. undulata is pleated and bluntly serrated at the margin (Table 2), unlike C. hayamensis without pleat margin (Yamada 1941).
Arakaki et al. (2011) described two new species, C. concepcionensis and C. macrostiolata, which exhibit interspecific divergences of rbcL with their close sister taxa, 4.0% with C. obtusifolia and 2.5% with C. atrosanguinea, respectively. Our interspecific divergences of rbcL are from 0.6-0.8% (between C. serratifolia sp. nov. and C. undulata sp. nov.) to 1.4-1.5% (between C. japonica and C. cartilaginea sp. nov.), which divergences are insufficient to distinguish the species (Arakaki et al. 2011). Otherwise, the interspecific divergences among our specimens in COI-5P ranged from the minimum 2.8–3.3% (between C. serratifolia sp. nov. and C. undulata sp. nov.), to the maximum 8.8% (between C. hayamensis and Callophyllis mollitia sp. nov.). Clarkston and Saunders (2013) defined that COI-5P shows a proper divergence to distinguish Callophyllis species efficiently, which 2.6–10.45% between collections from the northeastern Pacific. Although our rbcL sequences of Callophyllis exhibit relatively less interspecific divergences than 2.0%, our five new species are sufficiently supported by the complement of COI-5P analysis and morphological observations to recognize as a cryptic new species. Clarkston and Saunders (2013) emphasized the conjunction of key characteristics based on the morphology typically used for species delimitation and molecular data to identify many Callophyllis species exhibiting the high morphological variability (Abbott and Norris 1966). The morphological species boundaries for our taxonomic conclusion regarding Callophyllis species from the northwestern Pacific is guaranteed as a reliable criterion by conjunction with molecular evidence.
Abbott and Norris (1966) suggested that the number of carpogonial branches per supporting cell appear to differentiate two evolutionary lineages within Callophyllis. Clarkton and Saunders (2013) emphasized that mono- vs. polycarpogony has potential for the accurate definition of species entities and diversity, and that it is necessary to find a diagnosis that could be present in all of the life history of Callophyllis species. The mono- or polycarpogony of many Callophyllis species remain unrevealed as of yet, except several species from the Pacific coast of both America and Australia, including the generitype C. variegata (Norris 1957, Abbott and Norris 1966, Womersley and Norris 1971, Arakaki et al. 2011, Clarkston and Saunders 2013). The mono- or polycarpogony of Callophyllis species from the northwestern Pacific are not defined in detail in the present study. Further study of the female reproductive structures of Callophyllis should be carried out preferentially to find the diagnosis of Callophyllis species and to demonstrate the phylogenetic relationship of two evolutionary lineages. Up to now, several geographically small-scale studies of Callophyllis have been conducted in the southeastern Pacific (Arakaki et al. 2011, Lin et al. 2012) and the northeastern Pacific (Harper and Saunders 2002, Clarkston and Saunders 2013), whereas there has been no study in western Pacific region. Like the concept that geographically small-scale studies are a necessary for the first step towards a global assessment of Callophyllis (Abbott and Norris 1966), we expect that the present study will provide a basis for determining species diversity and understanding the phylogeographic relationships of Callophyllis from the Pacific Ocean.