Ascophyllum nodosum (L.) Le Jolis is the host of a complex symbiotic system that includes an obligate endophytic ascomycete, Mycophycias ascophylli (Cotton) Kohlmeyer & Volkmann-Kohlmeyer, and the obligate, epiphytic red alga, Vertebrata lanosa (L.) Christensen (Garbary and Deckert 2001). V. lanosa is, in turn, associated with the host-specific, red algal parasite Choreocolax polysiphoniae Reinsch (Irvine 1983). These are the four core members of a symbiosis that also involves the obligate, but not host-specific, epiphyte, Elachista fucicola (Velley) Areschoug (also on Fucus spp.), and the chironomid insect, Halocladius variabilis (Staiger), which is typically associated with E. fucicola (Garbary et al. 2009a, Tarakhovskaya and Garbary 2009, Brown et al. 2013). Considerable progress has been made in understanding the geographic and ecological distributions of these symbioses across the North Atlantic Ocean, as well as the anatomical and ultrastructural interactions of these associations (e.g., Lining and Garbary 1992, Deckert and Garbary 2005a, 2005b, Garbary et al. 2005b, Longtin and Scrosati 2009, Longtin et al. 2009, Toxopeus et al. 2011, Garbary and Tarakhovskaya 2013).
From a physiological perspective, the interactions of A. nodosum and V. lanosa (hereafter referred to as Ascophyllum and Vertebrata, respectively) have been the most intensively studied. Given the host specificity, it was initially assumed that host specificity was based on a biochemical dependency, and there was considerable interest in the possible transfer of materials between Vertebrata and Ascophyllum. A variety of studies showed photosynthate and inorganic nutrient transfer between the symbionts (Citharel 1972, Harlin 1973, Penot 1974, Harlin and Craigie 1975, Turner and Evans 1977, Penot et al. 1993, Ciciotte and Thomas 1997). None of these studies, however, provided a strong physiological explanation for the association, in that movement of materials was in both directions, and rarely in excess of what was considered simple diffusion. Indeed, Pearson and Evans (1990, 1991), Garbary et al. (1991), and Longtin and Scrosati (2009) suggested that the association had a strong ecological basis in recruitment limitation provided by host surface features. These included the number of settlement sites on Ascophyllum associated with scars from receptacle abscission and herbivore surface wounds, as well as epidermal shedding (Garbary et al. 2009b).
In this study, we used pulse amplitude modulation (PAM) of chlorophyll a fluorescence to measure photosynthetic parameters of Vertebrata and Ascophyllum when isolated from each other, or when in combination, where the two species were either attached or not attached to each other. We evaluated a series of hypotheses: 1) that Vertebrata is parasitic on Ascophyllum and will have a negative impact on the photosynthetic performance of the host; 2) that the survival of Vertebrata is based on exudates from Ascophyllum; and 3) that maintenance of photosynthesis in Vertebrata requires it to be in direct contact (i.e., rhizoid penetration) with Ascophyllum so that transfer of materials can be facilitated from host to epiphyte.
All algal material came from Gullivers Cove in the Bay of Fundy of Nova Scotia, Canada (44°36.85′ N, 65°55.56′ W) (Fig. 1A), and experiments were conducted during November-December 2012. Gullivers Cove is an undeveloped area that was previously studied for algal diversity (Edelstein et al. 1970), and is currently used for commercial harvest of Palmaria palmata (L.) Weber & Mohr (Garbary et al. 2012a). Ascophyllum grew on the basalt bedrock characteristic of Nova Scotian shores facing the lower Bay of Fundy (Garbary et al. 2012a), and where fronds typically reach 1 m long (Fig. 1B). The site has a continuous bed of Ascophyllum through the mid intertidal zone from ca. 1.5 to 5 m elevation, and Vertebrata (Fig. 1C-E) was abundant at all but the extreme upper and lower portions of the Ascophyllum distribution.
We examined photosynthetic performance of Ascophyllum and Vertebrata by measuring the maximum quantum yield of photosystem II (PSII) [QY(II)max], a measure of the photochemical efficiency of PSII, and the relative rates of photosynthetic electron transport (rETR) at various light intensities. Both photosynthetic parameters were measured as changes in the PSII chlorophyll a fluorescence emission using a portable, PAM fluorometer (Diving-PAM; Heinz Walz, Effeltrich, Germany). The QY(II)max was estimated as:
QY(II)max = (Fm − Fo) / Fm = Fv / Fm
, where Fo is the minimal value of chlorophyll a fluorescence from PSII in dark-adapted thallus (10-15 min) when both photochemical quenching (qP) is at a maximum (i.e., at 100%) and non-photochemical quenching (NPQ) is at a minimum (i.e., at 0). Fm is the maximum value for chlorophyll a fluorescence from PSII measured from dark-adapted thalli during a non-modulated flash of light that fully reduces PSII and is called a saturating flash (SF). Fv, the difference between Fm − Fo, is called the variable fluorescence (Papageorgiou and Govindjee 2004). To obtain thalli in the dark-adapted state samples were placed in the ‘dark leaf clips’ (DIVING-LC; Heinz Walz) and dark-adapted for 10-15 min prior to taking the readings. Gain settings on the Diving-PAM were set at 1 and 4 for Ascophyllum and Vertebrata, respectively. Chlorophyll a fluorescence nomenclature follows Kromkamp and Forster (2003).
The intensity of the monitoring beam of the PAM fluorometer was 0.04 μmol photons m−2s−1 and the intensity of the SF was 10,000 μmol photons m−2s−1.
The QY(II)max measurements give information only about the photochemical efficiency of PSII. In order to investigate the effect of treatments on the photosynthetic electron transport capacity downstream from PSII we measured rETR at various light intensities using the rapid light curve (RLC) option of the Diving-PAM fluorometer. The RLC protocol used began with dark-adapted thalli and the thallus was the exposed to eight sequential light periods (10 s each) of increasing light intensity (24 to 1,341 μmol photons m−2s−1). The rETR was determined using the following equation:
rETR = QY(II)eff × PAR × 0.5
, where QY(II)eff (also called ΦPSII) is the effective (i.e., actual) quantum yield of PSII at given intensity of photosynthetically active radiation (PAR). QY(II)eff is dimensionless while the intensity of PAR has units of μmol photons m−2s−1. QY(II)eff is calculated, by the Diving-PAM, using the following equation from Genty et al. (1989):
QY(II)eff = (Fm′ − Ft) / Fm′
, where Fm′ is the maximum chlorophyll a fluorescence, during a SF, measured during exposure of the thallus to the given PAR intensity and Ft is the steady-state level of fluorescence under the given PAR intensity at time t. QY(II)eff is lower than QY(II)max because of the presence of qP and NPQ under continuous PAR. The factor 0.5 in the equation is used to account for the assumption that photons absorbed by the thallus are partitioned equally between PSII and PSI (see Genty et al. 1989 for derivation of the equation). To calculate the absolute ETR, rather than rETR, requires information on the fraction of photons incident upon the thallus that are actually absorbed by the photosystems.
The degree of qP, due to increasing reduction of the first stable electron acceptor in PSII, Quinone A (QA), with increasing PAR intensity is calculated by the Diving-PAM (Walz 1998) by the equation:
qP = (Fm′ − Fo) / (Fm′ − Fo′)
and the degree of NPQ using the equation:
NPQ = (Fm − Fm′) / Fm′.
To establish baseline values for the photosynthetic parameters of Vertebrata growing under natural conditions, we measured Fo, Fm, and QY(II)max (measured as Fv / Fm) in situ at low tide on November 23, when air temperature was about 13℃ and there was intermittent cloud cover. To establish a range of natural values in the intact symbiosis, and to ensure that the immersed condition was optimal for our experiments, we compared thalli of Vertebrata on the surface of Ascophyllum fronds that were partially desiccated (max of 3 h of exposure) (Fig. 1E) with thalli following re-immersion in seawater. Re-immersion was done by draping long Ascophyllum fronds with their associated Vertebrata into plastic dishpans filled with seawater collected from the water’s edge (9℃). The Diving-PAM “leaf clips” were attached to hydrated Vertebrata within one minute, and thalli were dark adapted for 10-15 min prior to taking measurements. Eight to ten values of photosynthetic parameters were taken at 15 min intervals. These data were obtained over about 2 h when incident light was measured with a meter (LI-250; LI-COR, Lincoln, NE, USA) with a cosine corrected detector. Irradiance varied from 615 ± 11.5 to 76.1 ± 0.6 μmol photons m−2s−1 (mean ± standard deviation [SD], with six replicate readings taken within 30 s). Light was measured at nine, 10-15 min intervals from 1:30 PM to 3:20 PM. The extensive variation in light was based on changes in cloud cover, and the declining angle of the sun, which eventually set below the horizon of an adjacent cliff. An initial 30 chlorophyll a fluorescence readings of QY(II)max were made of exposed thalli, and then all subsequent readings (n = 69) were done with thalli that were immersed in seawater.
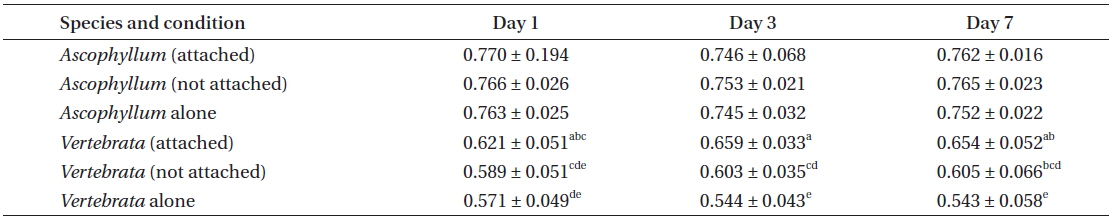
In an initial experiment, freshly collected fronds of Ascophyllum with and without epiphytic thalli of Vertebrata from the mid portion of the Ascophyllum zone (ca. 2.5 m elevation) were dissected to produce segments of Ascophyllum 14 ± 2 cm long that included an air bladder at either end, with or without epiphytic Vertebrata (A), and clumps of Vertebrata 2-4 cm long (B). Ten, 2-4-year-old segments of Ascophyllum (with or without Vertebrata), and ca. 25 clumps of Vertebrata (with or without Ascophyllum) were placed in each culture vessel. Duplicate 3.0 L plastic containers had one of four algal treatments: 1) Ascophyllum alone, 2) Vertebrata alone, 3) Vertebrata attached to Ascophyllum, and 4) Vertebrata not attached to Ascophyllum (Fig. 1F). Culture medium was seawater from Gullivers Cove. Seawater was collected every one-two days in 20 L plastic containers from the water’s edge, and used following a maximum of 48 h after collection. There was a 50% replacement of the unfiltered seawater each day to avoid potential nutrient depletion. Culture vessels were numbered and assigned algal condition using random numbers. Each day, culture vessels were rotated one position along the single culture shelf to minimize potential effects of position. Cultures were maintained at 14 ± 2℃ by opening and closing windows in the culture room. Cultures were continuously aerated, and illuminated with F32W T8 fluorescent bulbs with an irradiance of 40-90 μmol photons m−2s−1. This was supplemented during daylight hours with indirect natural light through the room windows (max. 10 μmol photons m−2s−1). Photosynthetic measurements were done from 8-11 AM each day with monitoring done on days 1, 3, 5, and 7. Data from day 5 was subsequently omitted because an extended power outage on day 4 produced aberrant values in all conditions on day 5, with subsequent recovery by day 7.
In a subsequent experiment (experiment 2), we omitted the condition, Ascophyllum alone, and monitored Fo, Fm, and QY(II)max daily for six days (n = 10 for each condition) in each of the triplicate culture vessels, but only for Vertebrata. On day 7 we also measured rETR and recorded associated quenching parameters (qP and NPQ) by carrying out RLCs on four samples from each culture vessel (total of n = 12 for each condition).
A wide range in quantum yield QY(II)max was measured in situ for Vertebrata in the intertidal zone of Gullivers Cove. Initially we evaluated naturally desiccated thalli on the surface of the Ascophyllum bed at low tide (Fig. 1B-E), and compared these to thalli immediately after re-immersion in seawater. Values of QY(II)max for partially desiccated thalli ranged from 0.121 to 0.718 (mean of 0.321 ± 0.177, n = 20). This was significantly lower (p < 0.05, Student’s t-test) than the quantum yield of thalli following re-immersion in seawater (0.587 ± 0.109, n = 69). Even here there was a wide range of values (0.210 to 0.710) and a clear peak between 0.601 and 0.650 (Fig. 2).
This experiment showed several clear photosynthetic responses on the part of Vertebrata with respect to the occurrence of Ascophyllum; however, there was no evidence for the converse. Accordingly, there was no significant difference in QY(II)max of Ascophyllum, regardless of whether Vertebrata was attached or not, or Vertebrata was present or absent in the same culture vessel (Table 1). The variation in QY(II)max for Ascophyllum in the three conditions after seven days was as follows: 0.758 ± 0.032 (Ascophyllum alone), 0.771 ± 0.021 (Ascophyllum with Vertebrata attached), and 0.767 ± 0.022 (Ascophyllum with detached Vertebrata; all values mean ± SD). The largest difference in QY(II)max between these means and the means after 24 h was 0.005, suggesting that there had been no deterioration in photosynthetic capacity of Ascophyllum over the seven day culture period.
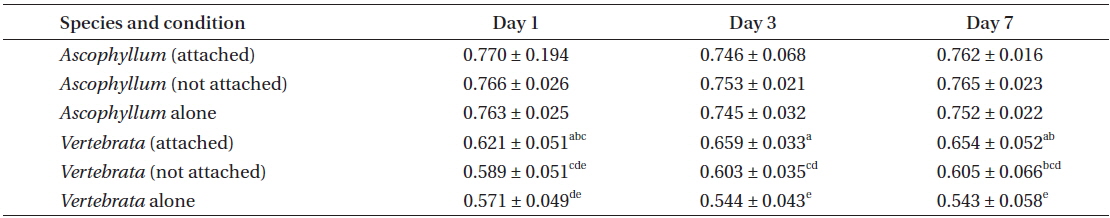
In the same experiment there were major differences in QY(II)max of Vertebrata in the three treatments, i.e., Vertebrata attached to Ascophyllum, Vertebrata with Ascophyllum, but not attached, and Vertebrata alone. Differences in the three conditions were apparent after 24 h and remained for the duration of the experiment (Table 1). A 2-way ANOVA evaluating QY(II)max of Vertebrata at days 1, 3, and 7 and three treatments (attached to Ascophyllum, detached with Ascophyllum, and alone) was significant (F ratio = 52.13; p < 0.001), with condition, and the interaction between condition and day significant at p < 0.05. Thus after 24 h attached Vertebrata had the highest maximum quantum yield (0.621 ± 0.047), and Vertebrata in the absence of Ascophyllum had the lowest QY(II)max (0.571 ± 0.049), with detached Vertebrata with Ascophyllum being intermediate between the other values (0.589 ± 0.051). Over the seven day experimental period there was no significant decline in QY(II)max of Vertebrata when attached to Ascophyllum (p = 0.616). Tukey post hoc tests showed that QY(II)max of Vertebrata attached to Ascophyllum was always significantly higher than Vertebrata alone, with the detached thalli (but in the same culture vessel) being intermediate between the other conditions, with considerable overlap in values (Table 1).
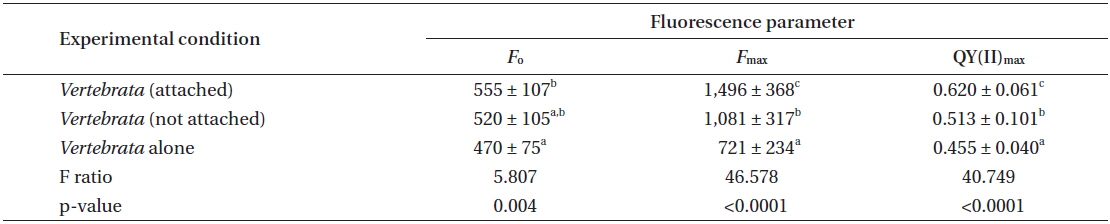
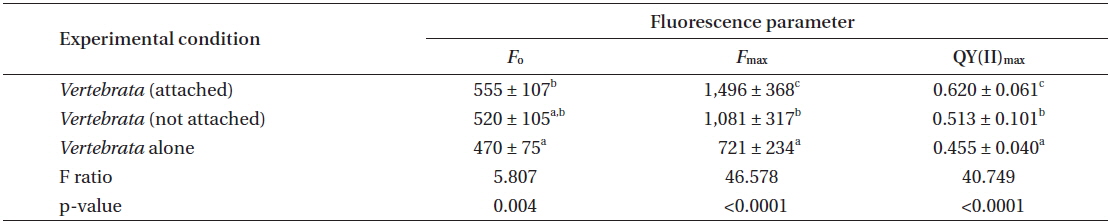
Experiment 2 evaluated only the performance of Vertebrata in the three treatments. The same primary result was obtained in this repeated experiment with respect to the QY(II)max of Vertebrata when alone, or either attached or not to Ascophyllum, but present in the same culture vessel as Ascophyllum. After seven days (Table 2), Vertebrata alone had the lowest QY(II)max (0.455 ± 0.040), Vertebrata attached to Ascophyllum had the highest QY(II)max (0.620 ± 0.061), and Vertebrata when separated (but in the same culture vessel as Ascophyllum) was intermediate (0.513 ± 0.100). The one-way ANOVA was highly significant (Table 2). The chlorophyll a fluorescence parameters of Fo and Fm showed the same trends as QY(II)max although the mean of Fo for Vertebrata (520 ± 105) when not attached (but in the same culture vessel) were intermediate but not significantly different from the other culture conditions (Table 2).
The values for QY(II)max between day 1 and day 7 for attached Vertebrata (0.631 ± 0.053 and 0.633 ± 0.062, respectively) were not significantly different (Student’s t-test, p = 0.89) despite significant declines in both Fo and Fm over the same time period in this condition (0.727 ± 0.152, 0.555 ± 0.107 and 2,023 ± 529, 1,496 ± 368 for Fo and Fm, respectively, Student’s t-test p < 0.001 for both). These declines (0, 23, and 26% for QY(II)max, Fo, and Fm, respectively) were generally lower than the equivalent reductions for Vertebrata alone (21, 33, and 49%, respectively) and Vertebrata with Ascophyllum, but detached (23, 20, and 46%). All of these declines from day 1 to 7 in Fo, Fm, and QY(II)max in Vertebrata alone and Vertebrata detached were highly significant (Student’s t-test, p < 0.001). The declines in these parameters may be based on a reduction in pigmentation (not measured), in that Vertebrata thalli in all conditions appeared to be a lighter red.
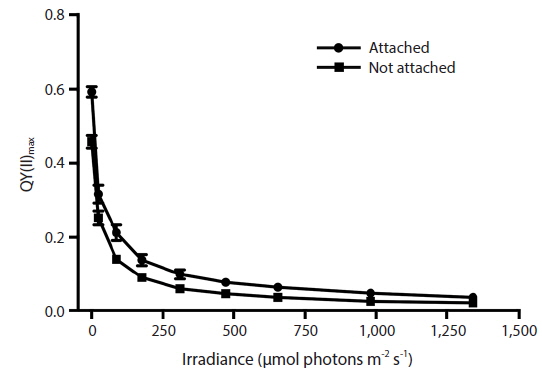
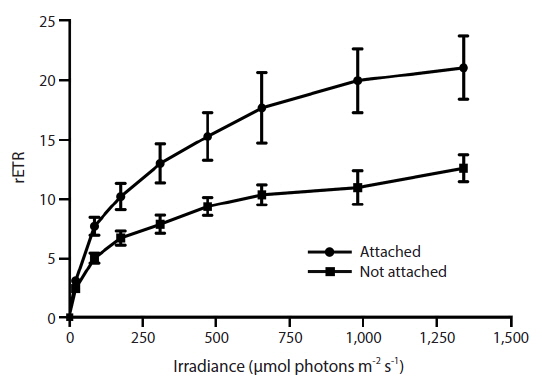
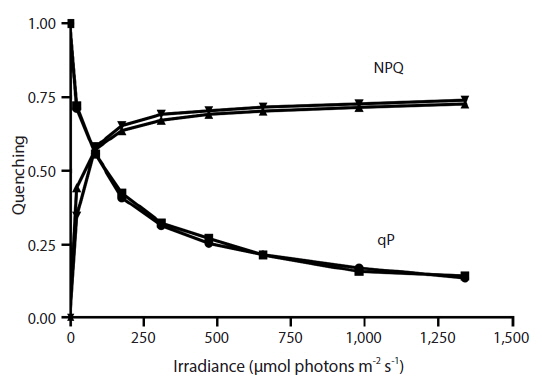
RLC for the determination of rETR at various intensities of PAR were only done with Vertebrata, either when alone or attached to Ascophyllum. For each RLC the first Diving-PAM measurement provides the value Fv / Fm, the estimate of QY(II)max, because the thalli were dark-adapted. Subsequent Diving-PAM measurements provide values of QY(II)eff, because the eight measurement subsequent to the first are obtained with the thallus now under illumination, from 24 to 1,341 μmol photons m−2s−1 (Fig. 3). With each subsequent light period during performance of the RLC the value of QY(II)eff decreases (Fig. 3) because of the increasing degree of quenching by qP and NPQ. The values for attached Vertebrata for QY(II)eff were significantly higher at each irradiance than those Vertebrata grown alone (Student’s t-test, p < 0.05) (Fig. 3). The difference of in QY(II)eff values for the two treatments, while of smaller absolute values with increasing irradiance, showed an increase in percent difference from ca. 20% at low irradiances to ca. 40% at high irradiances. Over the range of PAR intensities evaluated, rETR reached maxima of 21.1 ± 9.2 and 12.6 ± 3.9 (mean ± SD) at 1,341 μmol photons m−2s−1 for Vertebrata attached and Vertebrata grown alone, respectively (Fig. 4). Mean values of rETR in attached thalli were 21% to 45% higher in Vertebrata when attached to its host and this was significant at almost all irradiances (p < 0.05 Student’s t-test, except at 24 μmol photons m−2s−1 where p = 0.052).
The degree of NPQ was the same whether Vertebrata was grown alone or grown attached to its host Ascophyllum, and this was the case for all PAR intensities (Fig. 5). There was no significant difference in mean values at any irradiance. qP for the two conditions was also similar, and the curves were strongly overlapping through the range of PAR intensities (Fig. 5).
Photosynthetic parameters, determined by analysis of changes in chlorophyll a fluorescence emission from PSII have provided useful data on the relationship between Ascophyllum and Vertebrata. For the first time we have demonstrated a conclusive physiological basis for this symbiotic association. With regard to our initial hypotheses we have demonstrated that: 1) photochemistry of PSII [reflected in the value of QY(II)max] of Ascophyllum is not negatively affected by the presence of Vertebrata (Table 1), and thus there is no evidence of a parasitic interaction with respect to this important aspect of photosynthesis; 2) photosynthetic parameters are partially maintained when Vertebrata is separated from the host, but in the same culture, thus suggesting a role for diffusible substance from the host (Tables 1 & 2); and 3) long term maintenance of photosynthetic processes requires permanent attachment to Ascophyllum (Figs 3 &4).
The values for QY(II)max we obtained for Ascophyllum (i.e., means of 0.74 to 0.77) (Table 1) are among the best for brown algae (cf. Garbary and Kim 2005, Kim et al. 2006, Kim and Garbary 2009). The maintenance of high values for maximum quantum yield in Vertebrata in control conditions (i.e., attached to Ascophyllum) in the laboratory (i.e., laboratory experiment 1, 0.608 ± 0.075; laboratory experiment 2, 0.632 ± 0.060; mean ± SD) were clearly higher than the attached, immersed samples measured in situ (i.e., 0.569 ± 0.123). These values for both Ascophyllum and Vertebrata confirm that the laboratory measures for quantum yield were not depressed based on limitations of our culture conditions.
Laboratory results from the RLCs of Vertebrata (Figs 3 & 4) gave very different results for rETR when it was attached to Ascophyllum or alone in a culture. We therefore expected to observe differences in qP and NQP quenching characteristics for thalli in the two conditions (Fig. 5). This anomalous result may be explained by the apparent loss of pigmentation in Vertebrata when cultured alone. Unfortunately, this loss of pigmentation was a subjective determination that could not be quantified because of loss of samples during a subsequent power outage.
The virtually obligate association between Vertebrata lanosa and Ascophyllum nodosum would have been observed by Linnaeus (1767) (as Fucus lanosus Linnaeus, not seen, cited from AlgaeBase) (Guiry et al. 2014). Despite extensive ecological studies of the interactions (e.g., Garbary et al. 1991, Levin and Mathieson 1991, Cardinal and Lesage 1992, Garbary and Deckert 2001, Scrosati and Longtin 2010), this is the first time that a physiological basis for the dependency of Vertebrata on Ascophyllum has been clearly demonstrated. While we cannot demonstrate an underlying mechanism for the photosynthetic interactions, our results show that photosynthetic activity of Vertebrata declines when it is not attached to its host (Tables 1 & 2, Figs 3 & 4). The extent of this reduction in terms of QY(II)max and rETR suggest that Vertebrata requires its host for long-term maintenance of photosynthesis.
We have experimentally examined only one set of interactions, i.e., between Ascophyllum and Vertebrata; however, we cannot separate the potential interactions of Vertebrata and the mutualistic fungal endophyte M. ascophylli (hereafter Mycophycias). Garbary et al. (2005a) showed that fungal hyphae of Mycophycias regularly colonize the endophytic rhizoids of Vertebrata that penetrate into the cortical tissue of the host. Consequently, distinguishing between possible transport of materials between Ascophyllum and Vertebrata, and between Mycophycias and Vertebrata, is difficult. Garbary et al. (1991) suggested that host specificity of Vertebrata was based primarily on ecological factors associated with the production and number of suitable settlement sites of the host (e.g., receptacle dehiscence scars) and the timing of spore production by Vertebrata (Garbary et al. 1991, Kaczmarska and Dowe 1997). Colonization sites also are produced by surface wounds (Longtin and Scrosati 2009, Scrosati and Longtin 2010).
Mycophycias is always present in Ascophyllum (Kohlmeyer and Kohlmeyer 1972, Garbary and Deckert 2001, Deckert and Garbary 2005a, Garbary 2009), and the fungal hyphae are dispersed throughout the host from holdfasts through to branch apices and receptacles (Garbary and Gautam 1989, Deckert and Garbary 2005a, 2005b, Xu et al. 2008). In all frond portions, hyphae form complex networks in which rings of hyphae surround host cells, particularly in the cortex. Garbary and MacDonald (1995) showed that zygotes of Ascophyllum grew faster and with a different morphology following colonization by Mycophycias. Subsequent experiments showed that zygotes of Ascophyllum were more tolerant of desiccation following infection by Mycophycias (Garbary and London 1995). The abundant penetration of Vertebrata rhizoids by Mycophycias suggests that analogous physiological performance of Vertebrata may be partly based on colonization of the red algal rhizoids by the fungus. An alternative explanation for the decline in photosynthetic performance of Vertebrata when separated from its host is that it is based on phenolic (or other exudates) from Ascophyllum. Pearson and Evans (1991) showed stimulation in rhizoid production of Vertebrata based on exudates from tissues of a variety of brown algae. While this might partially explain our photosynthetic results, it is not consistent with the host specificity of Vertebrata.
On the one hand, we have little evidence for a mechanism to explain the causal basis for the reduction of quantum yield and electron transport rates in Vertebrata following detachment from Ascophyllum. On the other hand, the latter is a known source of phytohormones (e.g., Tarakhovskaya et al. 2007), and is harvested and used in the manufacture of commercial extracts that have a variety of applications in animal husbandry and agriculture (review by Craigie 2011). Externally applied phytohormomes to embryos of Fucus vesiculosus modify photosynthetic processes (Tarakhovskaya et al. 2013), and this might also be the case for Vertebrata. The commercial extract from Ascophyllum can regulate phytohormone biosynthesis and accumulation in Arabidopsis (Wally et al. 2012). We speculate that one or more of the secondary metabolites (e.g., phytohormones or phenolics) from Ascophyllum may be responsible for the maintenance of photosynthetic function when Vertebrata is attached to its host. However, we cannot rule out the possibility that the interaction is based on a chemical interaction with Mycophycias via direct connections with the rhizoids of Vertebrata (Garbary et al. 2005a). The intermediate response of quantum yield of Vertebrata when detached from Ascophyllum, but maintained in the same culture vessel as Ascophyllum, is strongpo suprt for some diffusible substance produced by Ascophyllum as being required for maximizing photosynthetic processes of Vertebrata. The production of secondary rhizoids by Vertebrata when exposed to fucoid extracts (Pearson and Evans 1991) demonstrates that Vertebrata has complex metabolic interactions with brown algae, and equivalent interactions may be part of the photosynthetic responses.
What is the nature of the Vertebrata-Ascophyllum interaction? Rawlence (1972) showed that cell death occurred during penetration of the V. lanosa rhizoids into the host, and Garbary et al. (2005a) demonstrated a hypersensitive reaction of some host cells adjacent to the red algal rhizoids. These interactions suggest a parasitic interaction between epiphyte and host. However, our photosynthetic data show no decline in photosynthetic performance when Ascophyllum has abundant Vertebrata compared to when it is absent. Indeed, Gauna et al. (2011) suggested that, both in terms of growth and initiation of receptacles, that there was no reduction in performance of the Ascophyllum when Vertebrata was present, and that host growth may even be stimulated by the epiphyte. The limited cell death induced by the epiphyte is minor compared to the massive sloughing associated with host epidermal shedding that occurs at regular intervals (Garbary et al. 2009b, 2012b, Halat et al. 2013). Thus the interactions between these species would appear to be at least commensal in nature, and may even be mutualistic.







![Percent frequency distribution of maximum quantum yield [QY(II)max] of Vertebrata lanosa measured in situ at low tide in partially desiccated (n = 30) thalli and following re-immersion in seawater (n = 69).](http://oak.go.kr/repository/journal/16469/JORHBK_2014_v29n4_321_f002.jpg)