



Global warming is receiving a great deal of attention, while on the other hand rising global energy demand combined with increasingly uncertain geopolitical conditions are making the production of energy from existing non-renewable sources more difficult, costly and unpredictable (Rizzi et al. 2014). Algae offer a tangible promise to fulfill the need for an alternative source of energy. While growing algae outdoors is generally expensive, the cost of this method of cultivation can be greatly reduced by using wastewater as a medium for their growth (Ahrens and Sander 2010, Clarens et al. 2010, Smith et al. 2010, Park et al. 2011, Stockenreiter et al. 2012). Algae can also produce a variety of high value compounds such as biodiesel and biofertilizer, without contributing to atmospheric carbon dioxide (Borowitzka 1995, Olaizola 2003, Banerjee and John 2005, Banerjee and Kushwaha 2005).
Wastewaters have been underutilized for their nutrient value resulting in downstream ecological disruption (Walsh et al. 2005). As a general practice, wastewaters are treated chemically and physically to remove undesired substances. Use of chemicals in treating wastewaters has serious long-term environmental effects and is expensive (Walsh et al. 2005). Therefore, there is a need to have a treatment method that has low environmental impacts and is economical. One simple solution would be the use of algae for wastewater treatment which can overcome most of the limitations of chemical treatment and provide cost effective removal of nutrients mainly nitrogen (N) and phosphorus (P) from the water in a process that would create fuels while reducing high nutrient loads in downstream watersheds (Banerjee and Yadav 2009, Park et al. 2011, Pittman et al. 2011, Rawat et al. 2011). Many microalgae have been screened with potential for biodiesel production and nutrient removal from treated municipal sewage (Chisti and Yan 2011, Odlare et al. 2011, Fortier and Sturm 2012).
Bioenergy production from algae is promising because productivity is high per land area, producing almost 300 times more oil per acre than conventional crops (Laws et al. 1988, Craggs et al. 2011, Dalrymple et al. 2013). Also algal cultivation does not compete with food production, algae have very fast growth rates which permit several harvests within a short time frame, and algae production absorbs CO2 from the atmosphere. Lipid and carbohydrate rich algae are a superior feedstock compared to many biofuel crops, especially for biodiesel production (Lam and Lee 2012). Many algal studies and energy evaluations have provided varying results (Huntley and Redalje 2007, Bruton et al. 2009, Batan et al. 2010, Brennan and Owende 2010, Clarens et al. 2010, Chisti and Yan 2011, Sturm and Lamer 2011, Lam and Lee 2012, Ramachandra et al. 2013).
However, life cycle analyses (LCA’s) that consider all energy inputs to algae production indicate that fertilizer inputs may contribute to poor energy gain in algae cultivation including biodiesel production from microalgae produced in outdoor ponds (Lardon et al. 2009, Batan et al. 2010, Clarens et al. 2010, Smith et al. 2010, Stephenson et al. 2010, Park et al. 2011). Most of these studies have assumed that the algal ponds will be supplied with chemical fertilizers in order to meet the N and P requirements for algal growth (Smith et al. 2010, Park et al. 2011). It was found that algal biodiesel could actually generate greenhouse gas emissions and require significantly more energy inputs compared to conventional crops (Smith et al. 2010, Park et al. 2011). At the same time it has also been proposed that most of the environmental burden associated with algae could be removed if wastewater was used as a nutrient source (Smith et al. 2010, Park et al. 2011). Most LCA’s use lab scale data for productivity and lipid content to design virtual biofuel production facilities because limited information is available on algal productivities and N and P removal rates achieved in wastewater fed outdoor systems (Smith et al. 2010, Sturm et al. 2012). Use of treated wastewater as a fertilizer source for algal growth eliminates the energy used in producing chemical fertilizers while simultaneously reducing eutrophication through removal of nutrients (mainly N and P) from wastewater (Clarens et al. 2010, Smith et al. 2010).
Cultivating algae in open reactors that may be colonized by other algae and by herbivorous invertebrates presents challenges in terms of stability of production (Smith et al. 2010). It has been suggested that diverse algal assemblages may contribute to more productive and / or more stable open reactors because of a generally positive relationship among plant diversity, stability, and productivity (McCann 2000, Smith et al. 2010, Rooney and McCann 2012, Stockenreiter et al. 2012) but tests of this are lacking in algal production systems. In a small scale test (reaction wells), Stockenreiter et al. (2012) found that more diverse algal assemblages had higher lipid production. Because an abundance of planktivorous fish is often associated with suppression of herbivorous zooplankton (that naturally colonize outdoor tanks) and high standing crops of algae (Carpenter et al. 1985, 1995), it has been suggested that including planktivorous fish increases algal production (Smith et al. 2010, Sturm et al. 2012). In the single test of this principle, Sturm et al. (2012) found increased production when fish were present in wastewater fed open tanks stocked with algal assemblages collected from local ponds.
In the present study, open tank systems were used for algal biofuel production which integrates wastewater treatment and algal biomass production to test whether 1) diverse assemblages of algae are more resistant to invasion and have greater, more stable productivity (mass, lipids, fatty acid methyl ester [FAME]) than low diversity algal assemblages, 2) inclusion of planktivorous fish increases stability and productivity of these open tank systems, and 3) greater algal diversity and / or the presence of planktivorous fish increase the removal of nutrients (N & P) from wastewater.
Outdoor experiments were conducted at a City of Houston wastewater treatment plant at Bellaire Boulevard and 8 Loop West (29.70821° N, 95.56657° W) that ran from July to November. We used treated wastewater (post-clarifier) as the nutrient source for open tank bioreactors. There were twelve, 2,270-L (2.43 m diameter, 61 cm deep) open-top plastic tanks that were operated as continuous-flow reactors (seven day hydraulic residence time). Water was drawn from the top meter of the clarifier, passed through a 400-µm polyester filter, and put into the tanks. To maintain even mixing the tanks were continuously aerated using a fine bubbler diffuser (~10 L min−1). Each tank overflowed through a stand pipe into a collection system.
These bioreactors were used to grow different lipid rich algae and natural algal phytoplankton samples that could be processed to make biodiesel or biocrude. We ran experiments using three algal taxa (
Each tank was assigned a treatment combination (algal diversity: low algal diversity [individual monocultures only] vs. high algal diversity [all 3 monocultures plus the wild collected algae]; trophic structure: fish vs. no fish) in a full factorial design with 3 replicates. Algae were added one day after we began pumping wastewater into tanks (so overflow did not begin until 6 days after algae were added).
To test whether adding zooplanktivorous fish to open tank reactors will increase algal production by providing top down ecological control of algal grazers, 15 individual mosquito fish (
Throughout the experiment at intervals of 15 days, we sampled and measured response variables related to water quality and nutrient removal in the water being put into and being drained from tanks (including N and P) and algal production as total suspended solids (TSS, measured as dry mass).
A depth integrated water column sample was collected from each reactor for evaluation of dry weight, Chl
Chl
To analyze total lipids each sample was mixed thoroughly and 10 mL from each sample was pelleted via centrifugation. The pellet was resuspended in 8 mL of methanol, incubated at 65℃ for 1 h. Then 4 mL of chloroform was added and the mixture was incubated at 50℃ for 1 h. This process was repeated to recover additional lipids. The pooled solvents were transferred to a pre-weighed vial, and the solvents containing extracted lipids were evaporated overnight. The vial was re-weighed to yield a gravimetric weight of total extracted lipids. Extracted lipids were normalized to dry cell weight.
To analyze FAME conversion, 10 mL from each sample was pelleted via centrifugation. The pellet was resuspended in 8 mL of methanol and 1 mL of 10 N KOH, and incubated at 65℃ for 2 h with gentle mixing every 30 min. Then 0.5 mL of 24 N H2SO4 was added to each sample, and incubated for another 2 h with gentle mixing every 30 min. Four mL of hexane was added to each vial, mixed thoroughly, then phase partitioned via centrifugation. The upper hexane layer containing FAMEs, was transferred to a pre-weighed vial, and then evaporated overnight. The vials containing the recovered FAMEs were re-weighed to determine total FAME content. A >95% conversion of fatty acids to FAMEs was confirmed via thin layer chromatography. Extracted lipids were normalized to dry cell weight.
We examined the effects of algal cultivation on physical and chemical parameters (temperature, pH, dissolved oxygen [DO], total dissolved solids [TDS], nitrate-N, P) in repeated measures ANOVAs with categorical time variables and a single predictor (supply vs. tank).
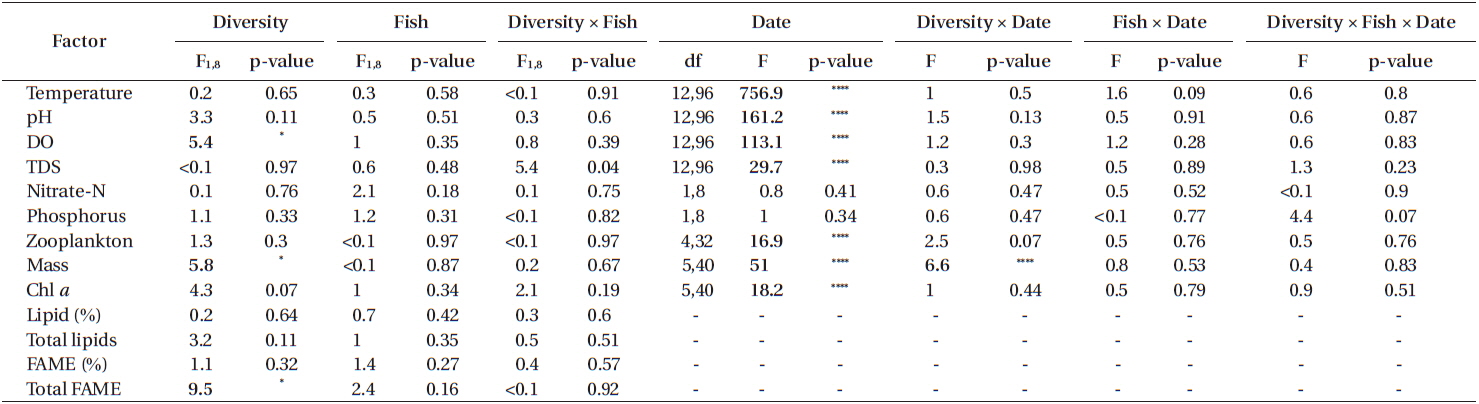
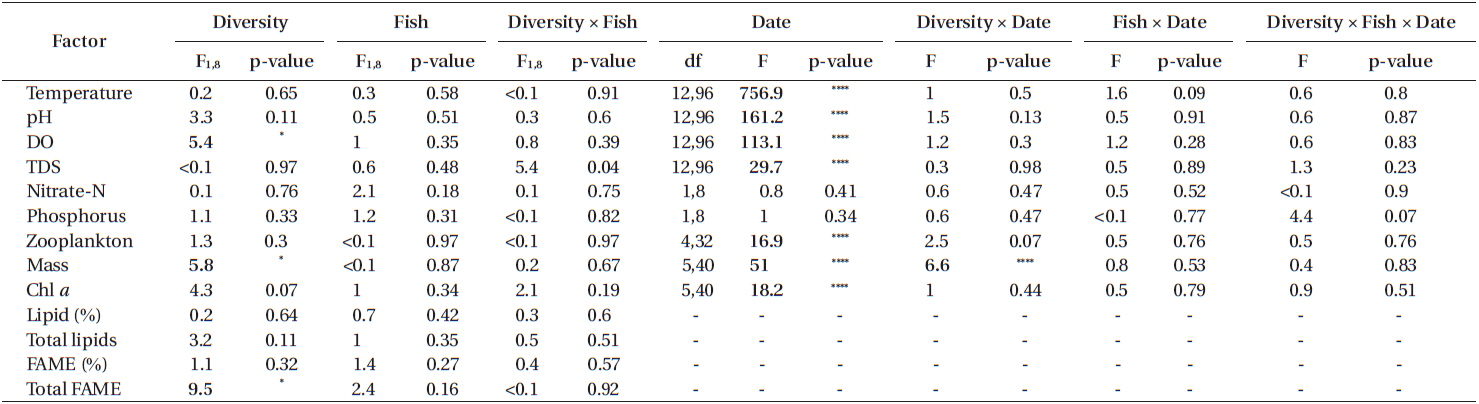
Another set of repeated measures ANOVAs was conducted that examined the effects of experimental factors on physical parameters, chemical parameters, algal production, and zooplankton abundance (that colonized naturally). The predictors were diversity (algal monoculture vs. diverse algal assemblage), fish (present or absent), and diversity × fish. Adjusted means partial difference tests were used to examine differences between treatments on a sampling date for significant treatment by time predictors.
ANOVAs were used to examine the effects of experimental factors on lipids (% of mass and amount) and FAME (% of lipids and amount).
Repeated-measures MANOVA was used to examine the effects of experimental treatments on algal community composition. This analysis had six response variables that corresponded to the taxonomic groups found in our censuses. Analyses were conducted using SAS version 9.0 (SAS Institute Inc., Cary, NC, USA).
>
Comparisons of tanks and supply water
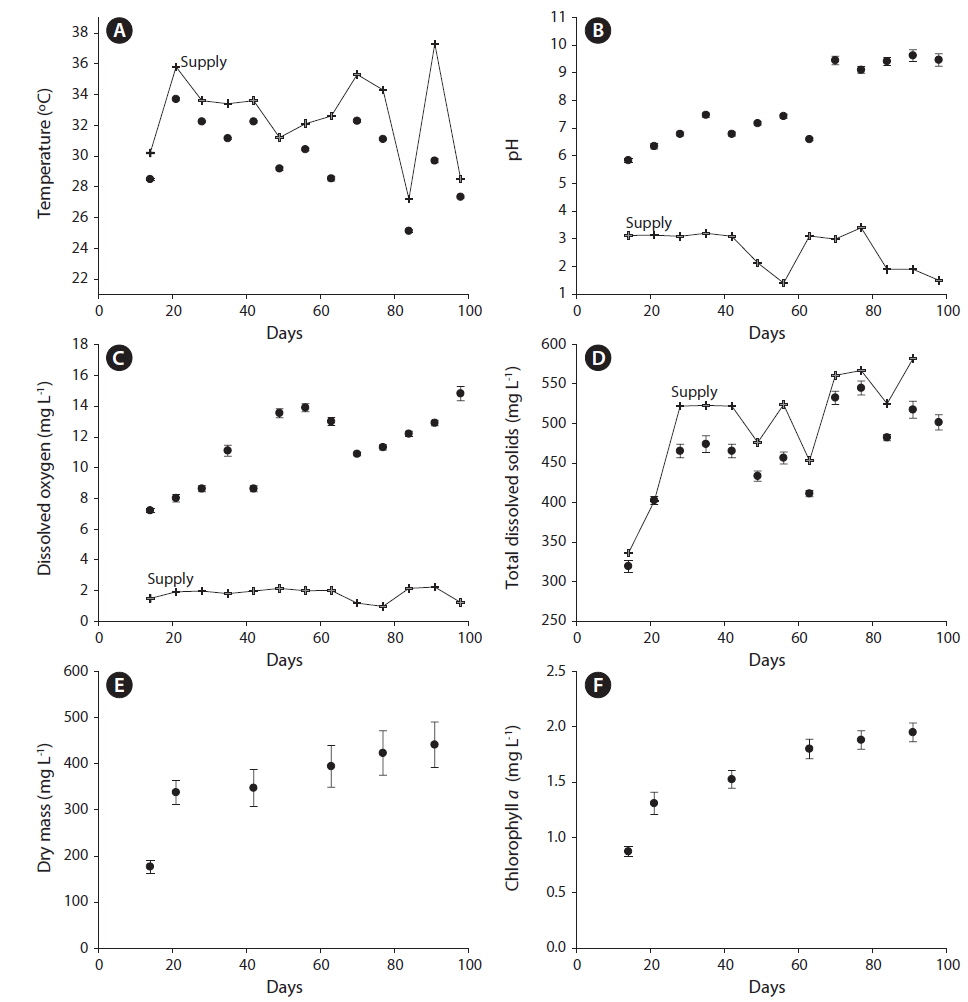
The water in tanks differed from the supply water for all physical and chemical characteristics we measured. Compared to supply water, the water in tanks had lower temperatures (tanks, 30.11 ± 0.03℃C; supply, 32.70 ± 0.12℃; F1,11 = 425.8; p < 0.0001) (Fig. 1A), higher pH (tanks, 7.80 ± 0.07; supply, 2.61 ± 0.23; F1,11 = 467.2; p < 0.0001) (Fig. 1B), higher dissolved oxygen (tanks, 11.25 ± 0.12 mg L−1; supply, 1.78 ± 0.41 mg L−1; F1,11 = 497.7; p < 0.0001) (Fig. 1C), and lower TDS (tanks, 457.85 ± 3.13 mg L−1; supply, 503.05 ± 11.40 mg L−1; F1,11 = 10.1; p < 0.01) (Fig. 1D). All of these parameters also varied significantly with sampling period (temperature: F12,132 = 250.93, p < 0.0001; pH: F12,132 = 10.39, p < 0.0001; DO: F12,132 = 9.11, p < 0.0001; TDS: F12,132 = 10.18, p < 0.0001) as did the magnitude of the differences between tanks and supply water (i.e., date by tank vs. supply interaction term) for all these parameters except TDS (temperature: F12,132 = 30.03, p < 0.0001; pH: F12,132 = 22.38, p < 0.0001; DO: F12,132 = 8.74, p < 0.0001; TDS: F12,132 = 0.21, p = 0.997).
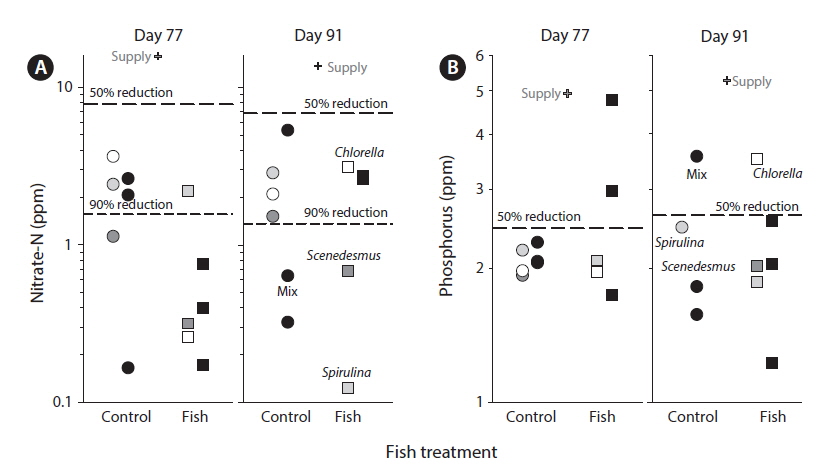
The supply water had relatively higher concentrations of nitrate-N, lower concentrations of P, and higher N : P on day 77 compared to day 91. Compared to supply water, the water in tanks had significantly lower concentrations of nitrate-N (F1,11 = 136.7, p < 0.0001) and phosphorus (F1,11 = 18.8, p < 0.01). Compared to the supply water, nitrate-N was reduced by 91% (supply, 14.74 ± 1.08 ppm; tanks, 1.60 ± 0.31 ppm) (Fig. 2A) and phosphorus was reduced by 53% (supply, 5.10 ± 0.65 ppm; tanks, 2.19 ± 0.19 ppm) (Fig. 2B). Neither nitrate-N nor P (in terms of the experiment-wide average of supply water and tanks) varied significantly between the two sampling periods (each p > 0.51). The reductions in nitrate-N and P from uptake were independent of sampling periods as well (each p > 0.48).
Experimental treatments had few effects on any chemical or physical characteristics. There were no significant effects of treatments alone or in interaction on temperature, pH, TDS, N or P (Table 1). DO was significantly higher in tanks seeded with algal monocultures (significant diversity effect) compared to those seeded with mixtures (Table 1). All of these chemical and physical characteristics varied significantly with time but there were no significant interactions of experimental treatments and time (Table 1).
The effects of experimental treatments on physical parameters of water, zooplankton abundance, and production of mass, lipids, and FAME
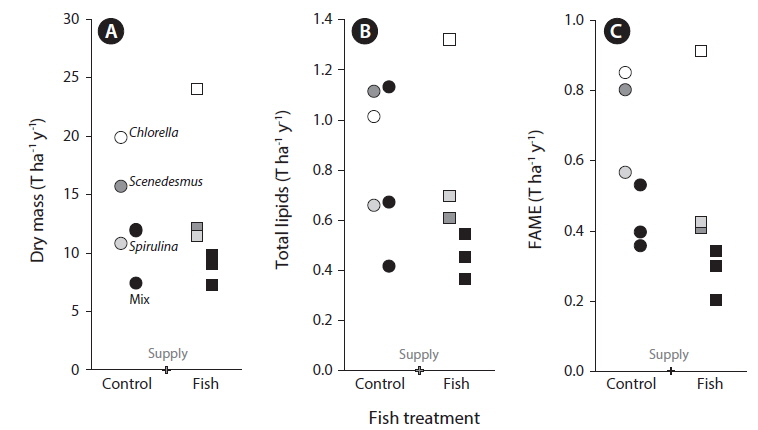
Dry mass (Figs 1E & 3A) but not Chl
Zooplankton abundance was independent of all treatments (Table 1, Fig. 4). Zooplankton abundance varied significantly with time but there were no significant interactions of time and treatments (Table 1). In the MANOVA, algal genus composition depended significantly on diversity (p < 0.0001) but not fish (p = 0.9227) or fish × diversity (p = 0.8761). Algal genus composition varied over time (p < 0.0001) but the effects of treatments did not vary with time (diversity × date: p = 0.3547; fish × date: p = 0.6833; diversity × fish × date: p = 0.6801). Contamination with other algal species was not noticed or detected in monoculture tanks other than the ones that were seeded into the tank (Fig. 4).
Contrary to some previous reports we found that our monocultures (
Compared to the other published study of open-tank wastewater fed reactors which was conducted in Kansas, USA (Sturm et al. 2012), our productivity in terms of lipids per liquid volume was, on average, three times higher (25.4 mg L−1 vs. 8.35 mg L−1). Our tanks were much warmer on average than those in Kansas. Our tanks averaged 30.3°C while the other study had temperatures of 16.4℃ during the start-up period and 10.6℃ during the stable operation period. If the higher temperature of our tanks is related to higher productivity, this suggests that warm locations such as Houston might be especially suitable for such an industry. In comparison, our supplied wastewater was lower in nitrogen (14.7 mg L−1 vs. 20.4 mg L−1), higher in phosphorus (5.1 mg L−1 vs. 3.5 mg L−1), and had a lower mass ratio of N to P (2.9 vs. 5.8). It is possible that these differences in nutrients also played a role in differences in productivity. However, pH and DO were similar and so differences in those factors are not likely responsible for differences in productivity. There also may have been less shading due to biomass accumulation because our tanks were half as deep. In this experiment, lab-grown selected algal strains were used to inoculate our tanks, along with wild isolates in the mix treatments, while the study in Kansas only used wild isolates. The use of cultured algae is important compared to wild isolates when one considers commercialization of the bioremediation process and biomass generation for biodiesel production. There were also differences in experimental methodology such as inoculation rates, timing of inoculation vs. filling, and days to first overflow after inoculation. Because the differences in productivity (and fish effects, see below) were large, understanding the causes of variation is important. The potential roles of different changes in water chemistry on algae, zooplankton, and fish on the results we found here cannot be disentangled without additional experimental factors.
Total lipid and FAME analysis showed high lipid production in the algal biomass of all treatments. Estimates of FAME predicted that almost 80% of the lipids could be converted into biodiesel (Fig. 3). The total FAME data obtained in this study is comparable to that obtained by Ramachandra et al. (2013) although the algal species and conditions were different then what we used. Lipid productivity (mass of lipid that can be produced per day) is dependent on the lipid content of the algal biomass. Algal biodiesel production will therefore be limited by the standing crop of the microalgae and also by the lipid content. Both the quality and quantity of lipids produced will vary with the identity of the algal species as well as the site specific growth conditions (Brennan and Owende 2010, Sydney et al. 2011, Yang et al. 2011, Stockenreiter et al. 2012). This variability probably reflects alteration in relative rates of production and utilization of storage lipids and modifications in the properties of cellular membranes (Rodolfi et al. 2009). Our lipid and FAME estimation results clearly show that it has high probability of converting to significant amounts of biodiesel. This is all the more probable as we have used algal species selected for high lipid content. Optimizing stress conditions to obtain the highest possible lipid yields in the cells may also be important. Nitrogen starvation has been shown to increase total lipid content in many algal species (Pulz and Gross 2004, García et al. 2006, Elser et al. 2007). In addition, nitrogen availability is a major factor determining the algal lipid class composition (Tedesco and Duerr 1989, Gordillo et al. 1998, Alonso et al. 2000). The high rates of nitrogen removal and low nutrient concentrations in discharge water suggest that either nitrogen or phosphorus could have been limiting nutrients in this study and suggest that it may be possible to obtain higher or lower lipid content depending on the nitrogen and phosphorus content of wastewater (Smith et al. 2010). It is possible that sampling at a finer temporal resolution might have provided more power to examine effects of treatments.
The low concentrations of available nutrients in tanks may also be responsible for the stable monocultures that were apparently resistant to invasion by other types of algae. If monocultures inoculated in the wastewater fed open bioreactors took up available nutrients at a very fast rate creating a nutrient deficient environment, it is possible that this may have prevented the establishment of contaminating species in our tanks (Shurin et al. 2013). The inoculation rates here were low (about 1 : 10,000 of the Chl
The amounts of nutrients in water discharged from the tanks (after algal removal) were much lower than those in supplied wastewater. Indeed, algae cultivation removed more than 90% of the nitrogen and more than half of the phosphorus from supplied wastewater (Fig. 2). In comparison to the study in Kansas, the removal of nitrogen was much higher (91% vs. 19%) but the removal of phosphorus was only slightly higher (57% vs. 46%) (Sturm et al. 2012). In terms of concentrations in the discharge water after algal removal, in this study the concentrations of nitrogen were much lower (2.3 mg L−1 vs. 16.5 mg L−1) while phosphorus concentrations were comparable (2.0 mg L−1 vs. 1.9 mg L−1). Overall, the high rates of nutrient removal found here were consistent with high algal production perhaps in combination with a similar minimum available phosphorus concentration. The results (Fig. 2) showed variation in rates of nutrient removal but this variation did not depend on treatments.
There was no positive effect of algal diversity on any variable which is consistent with stable, highly productive monocultures. Algal researchers typically prefer monoculture production and go to considerable lengths to maintain the purity of their cultures but it has been suggested that monocultures will not be stable or productive in open tank systems (Smith et al. 2010). The higher productivity of monocultures than diverse mixtures that include those species (“underyielding”) is not predicted by well-accepted models of diversity and productivity (Loreau et al. 2001). Diversity is known to affect productivity either through sampling effects in which diverse communities are more likely to include the most productive species or niche differentiation and complementarities in resource utilization (Shurin et al. 2013). The sampling effect leads to polycultures equivalent to the most productive monocultures and niche complementarities leads to polycultures more productive than every monoculture (“overyielding”). One mechanism that can lead to underyielding is direct negative interactions among algal species. In fact, allelopathic interactions are an important factor in determining species presence and abundance within planktonic communities. Microalgal allelopathy in certain groups of algae like the diatoms and cyanobacteria may negatively affect the performance of each other while existing as a mixed community in the same environment (Bacellar Mendes and Vermelho 2013). This is consistent with our study in which
In contrast to the Kansas study in which the presence of fish significantly increased Chl
The results of this pilot experiment are not equivalent to establishing long-term, large-scale, commercial efforts. However, they add to the set of demonstrations that algal biomass can be grown in open tank bioreactors using wastewater as a nutrient source for biodiesel production. Perhaps the most interesting aspect of this study was the successful cultivation of stable and highly productive monocultures in open tanks which challenges the idea that wastewater-fed open pond systems will quickly become contaminated and therefore are not suitable for commercial production systems. Rates of nutrient removal, especially nitrogen, from wastewater were also much higher then what has been previously reported. The marked differences between this study and others motivate additional studies to examine the factors which underlie these large differences in productivity and nutrient removal including variation in wastewater chemistry, temperature, and naturally recruiting predators of zooplankton.


![Algal species composition over the course of the experiment. For monocultures, each data point represents an individual tank without (A, C & E) or with fish added (B, D & F). For tanks that received a diverse mixture of algae, each data point is the average of three tanks without (G) or with fish added (H). Circles indicate strains included in monocultures (white, Chlorella; dark gray, Scenedesmus; gray, Spirulina), black triangles indicate zooplankton, and diamonds indicate algae not included in monocultures (dark gray, Aphanocapsa [cyanobacteria]; white, Chlamydomonas [Chlorophyta]; gray, Euglena [Euglenoids]; light gray, diatoms [Dinophyta]).](http://oak.go.kr/repository/journal/16202/JORHBK_2015_v30n1_67_f004.jpg)