Removal of sericin from silk fibers improves the luster and feel of silk textiles, and so, sericin was considered a useless protein. However, silk sericin was recently reported to exhibit inhibition of ultraviolet B-induced apoptosis in human skin keratinocytes (Dash
Sericin film can be used in various applications. However, sericin film is too brittle, showing poor mechanical properties that restrict its application in various biotechnological fields. Recently, Jo and Um (2015) reported that formic acid casting can induce a two-fold increase in the mechanical properties of sericin film. However, the mechanical properties of sericin film need to be enhanced to a greater extent for biomedical and cosmetic applications.
Silk is produced by the silkworm (
In the present study, nine different
Silk cocoons of the same silkworm variety used in the previous study were selected (Chung
Preparation of silk sericin and its film has been described in the previous study (Jo and Um, 2015). Briefly, sericin was extracted by immersing silk cocoons in water at 120℃ for 30 min by using an autoclave (JSAC-60, JSR, Japan). The liquor ratio was 1:25. The extracted sericin solution was filtered using a non-woven fabric and dried at 80℃ in a drying oven to obtain solid sericin.
Solid sericin was dissolved in 98% formic acid at 55℃ to prepare 3% (w/w) sericin formic acid solution and then poured into a petri dish. Then, it was dried in a hood at room temperature to obtain sericin film.
>
Measurement and characterization
Amino acid composition of sericin powder was determined using an amino acid analyzer (L-8900; Hitachi, Japan). The sericin samples were added to 6N HCl solution, and the solution was treated at 110℃ for 24 h to hydrolyze the sericin samples before the amino acid analysis. Then, 0.3% (w/w) of the sericin formic acid solution was used for the rheological measurements. The complex viscosity was measured using a rheometer (MARS III, HAKKE, Germany) with a cone and plate geometry with an angular frequency of 0.1–10 rad/s at 25 ℃. The radius and angle of the cone were 60 mm and 1°, respectively. FTIR (Nicolet 380, Thermo Fisher Scientific, USA) spectra of the sericin films were obtained using the ATR method.
To evaluate the mechanical properties of sericin film, breaking strength, breaking elongation, and initial Young’s modulus were obtained using the Universal Test Machine (OTT-03, Oriental TM, South Korea). The tensile tests were performed using a3-kgf load cell at an extension rate of 10 mm/min for the sericin film. The film sample had a length of 80 mm and a width of 5 mm. The gauge length (measuring length of the sample) was 30 mm. All samples were preconditioned at 20℃ and 65% (RH), and seven sericin films from each silkworm variety were tested.
>
Amino acid composition of sericin
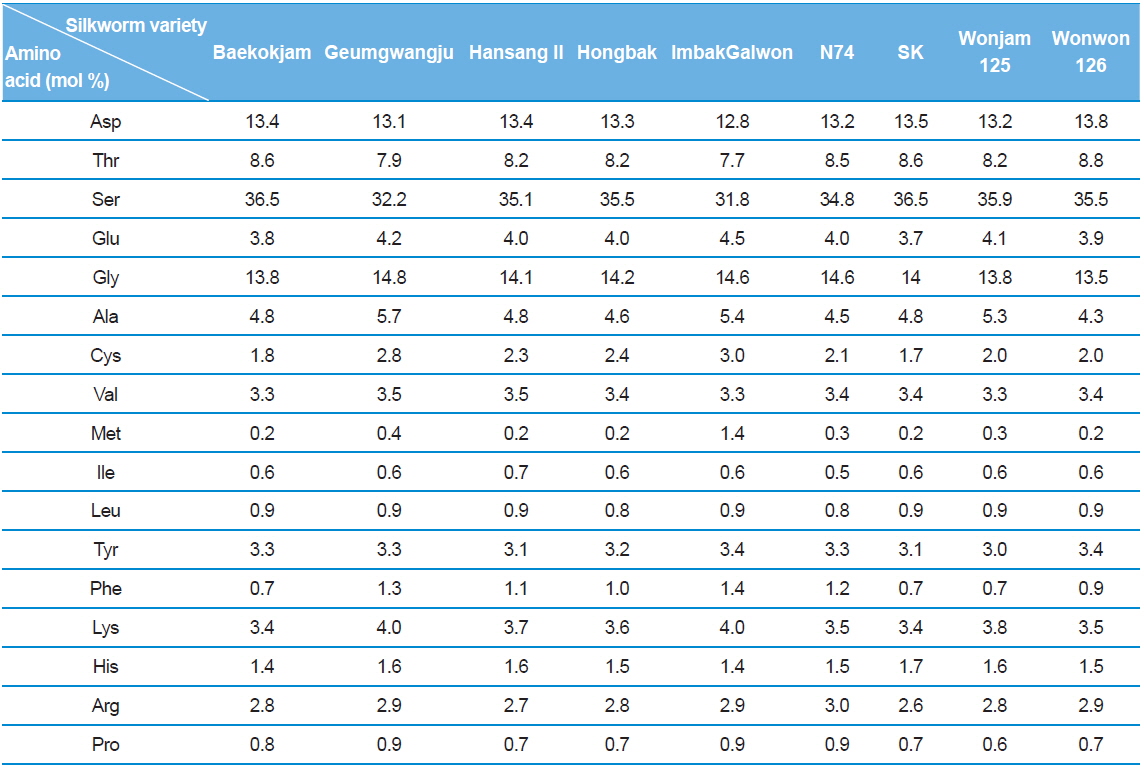
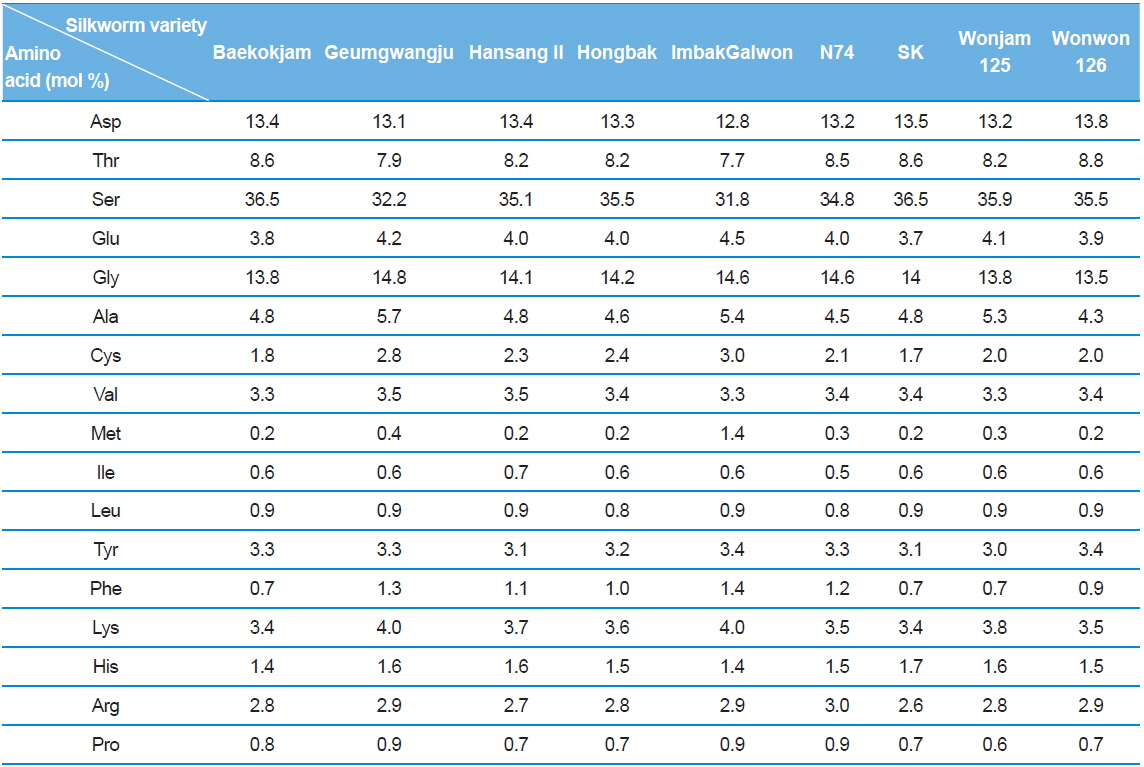
Silk sericin is a protein polymer that consists of various amino acids. The amino acids have polar and nonpolar groups, depending on the type of amino acid. Thus, the character and composition of an amino acid affect the properties of sericin, such as hydrophilicity and solubility in water. The amino acid composition of sericin produced from different silkworm varieties was evaluated (Table 1).
[Table 1.] Amino acid composition of silk sericin produced from different silkworm varieties
Amino acid composition of silk sericin produced from different silkworm varieties
Interestingly, Gumgwangju (32.2%) and Imbakgalwon (31.8%) showed lower serine contents than the other varieties (34.8–36.5%). Other than that, no significant differences were observed among the varieties.
>
Rheological properties of the sericin solution
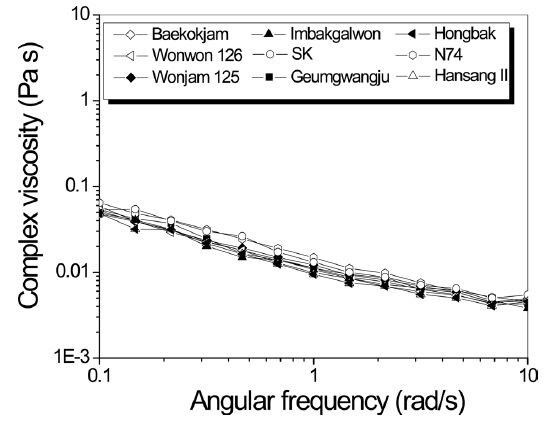
Rheology of silk solution has been studied because it provides indirect information on MW and affects the processability of silk solution, including wet- and electro-spinning (Kim and Um, 2014; Cho
Therefore, to obtain indirect information on MW and rheology behavior of sericin, complex viscosity was measured as a function of angular frequency (Fig. 1). Regardless of the silkworm variety, all sericin formic acid solutions showed similar viscosity values. This indicates that the MW of sericin from different silk varieties is similar.
Considering that regenerated SF showed somewhat different MW and solution viscosity according to the silkworm variety (Chung
>
Molecular conformation and mechanical properties of sericin film
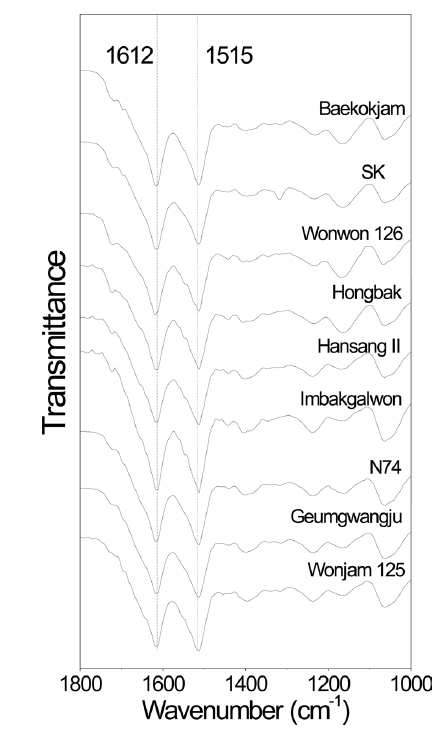
Molecular conformation of silk proteins has been examined because it affects the properties of silk proteins (Um
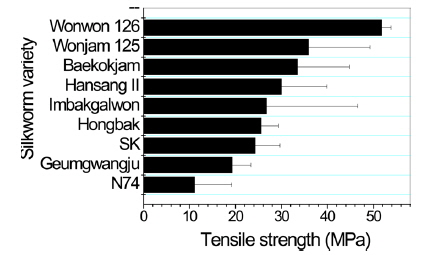
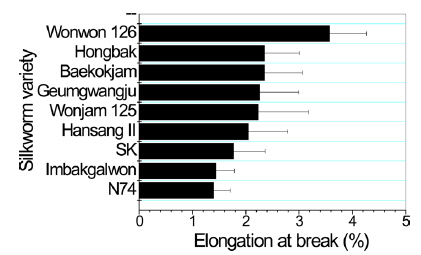
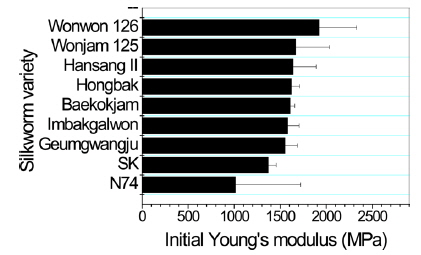
Poor mechanical properties of sericin film have prevented the use of sericin film in industrial fields. Therefore, breaking strength, elongation, and initial Young’s modulus of sericin film obtained from different silkworm varieties were measured to examine the effect of silkworm variety on the mechanical properties of sericin film.
In case of breaking strength (Fig. 3), Wonwon 126 showed the highest strength among the samples. Geumgwangju and N74 showed the lowest values among the sericin films. For elongation, a similar trend was observed (Fig. 4). In case of initial Young’s modulus, Wonwon 126 and N74 showed the highest and lowest values, respectively, among the samples. Wonwon 126 showed the best mechanical properties for sericin film, whereas Geumgwangju and N74 displayed the worst properties. These results indicate that silkworm variety significantly affects the mechanical properties of sericin film. For example, Wonwon 126 showed a five-fold higher breaking strength than N74.
Although the mechanism underlying the different mechanical properties of sericin film on the basis of silkworm variety could not be elucidated, it is interesting that Wonwon 126 showed better mechanical properties than Baekokjam (standard).
In this study, the effect of silkworm variety on the structure and properties of sericin was examined. Although the amino acid composition and solution viscosity of sericin were very similar among the silkworm varieties, the mechanical properties of sericin film were remarkably different depending on the silkworm variety. Considering that the poor mechanical properties of sericin film has restricted its application in biotechnological fields, this finding shows that an optimum silkworm variety can be a good solution for improving the mechanical properties of sericin film. Because silkworm variety was found to affect the properties of sericin, a more detailed and related study on silkworm varieties should be performed in the future to identify better silk protein materials for various applications, including biomedical and cosmetic applications.