The chub mackerel,
The chub mackerel is a candidate for aquaculture development in Korea, with high market prices due to a strong demand for live aquaculture products. Although artificial rearing techniques of the chub mackerel have recently been reported in Korea, the early development of the scombrid larvae is poorly understood, primarily due to difficulties in rearing the larvae. Culturing of the early stages is considered the most critical phase for successful aquaculture (Tanaka, 1973). In particular, a lack of information of the nutritional requirements of the fish larvae and the development of their digestive system is thought to play a major role in the limited success of larval culture of many species. Hence, to develop successful aquaculture methods, species-specific information on the early development of larvae is required to provide the best feeding regimes to match the ontogenetic status of the larvae. However, there is no documented ontogeny of the digestive system in
Fertilized eggs of chub mackerel were obtained from a broodstock maintained by Gyongsangnam-do Fisheries Resources Research Institute, Korea and stocked in a 20-ton rearing tank. The water was maintained at an average temperature of 19.1 ± 2.2°C. Rotifers were fed immediately after hatching until 19 DAH, and
Eighty individuals were collected from hatching on a daily basis for 35 days from 31 May 2011 until 5 July 2011. Fifty individuals were fixed in 10% natural formaldehyde for morphological investigation (Olympus DP20, Olympus Co., Japan). Total length (TL) and standard length (SL) were measured to the nearest 0.1 mm. The developmental phases were classified into 5 stages (the yolk-sac larvae, 0–2 DAH; preflexion larvae, 3–12 DAH; flexion larvae, 13–16 DAH; postflexion larvae, 17–26 DAH; and juvenile, 27–35 DAH) based on Kendall et al. (1984). The criteria for classification were as follows: the yolk-sac larval stage, a transitional stage (from hatching to yolk-sac absorption); the larval period, divided into three phases (preflexion phase: first feeding to onset of notochord flexion: flexion phase: to completion of notochord flexion; postflexion phase: to completion of fin formation) depending on the flexion of the notochord that accompanied the hypochordal development of the homocercal caudal fin, and the juvenile stage (completion of fin ray counts and beginning of squamation until the fish entered the adult population or attained sexual maturity).
Individual fish were placed on pre-weighted pieces of foil and dried at 60°C for 48 h to obtain the dry weight measurements. The remaining fish were fixed in Bouin’s solution for 24 h and preserved in 80% ethanol for subsequent histological preparation. The larvae were dehydrated and embedded in paraffin. Serial sections were made with a microtome at a thickness of 5–7 μm. Sections were stained with Mayer’s hematoxylin and eosin and then observed under a microscope system (Olympus BX51, Japan, and Leica DM2500, Germany).
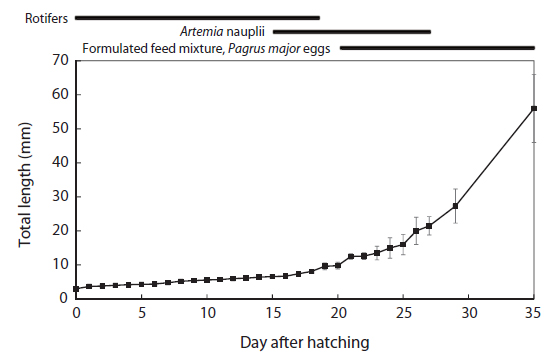
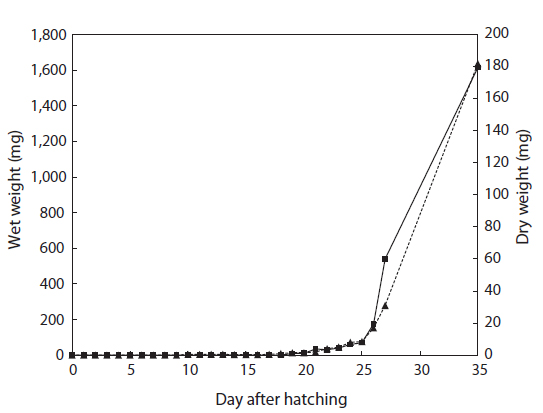
The total length of larval fish averaged 3.35 ± 0.14 mm just after hatching, 3.91 ± 0.20 mm at first feeding (3 DAH), 5.91 ± 0.45 mm at the flexion phase (13 DAH), 7.09 ± 0.61 mm at the postflexion phase (17 DAH), 21.46 ± 2.45 mm at the juvenile phase (27 DAH), and 56.64 ± 9.27 mm at the experimental end date (35 DAH). The total length of the chub mackerel larvae increased markedly from the postflexion phase to the juvenile phase (Fig. 1). The wet and dry weights were 4.6 and 0.4 mg, respectively, at 17 DAH; by 27 DAH, the wet and dry body weights had increased >60 fold, to 277.1 mg and 59.1 mg, respectively (Fig. 2). The average growth rate during 35 days of rearing was approximately 1.52 mm/day.
>
Development of the Digestive System
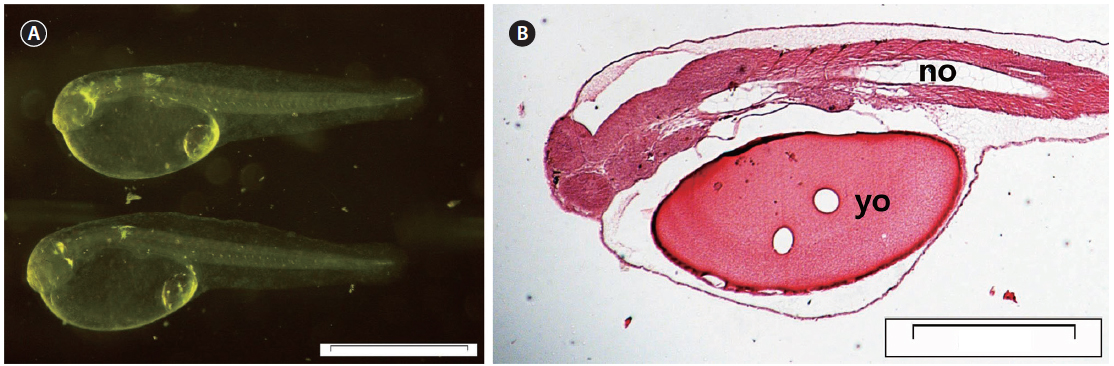
At 0 DAH (3.35±0.15 mm TL), the mouth and anus were unopened, and the melanosome in the eyes was not yet colored (Fig. 3A). Most of the body cavity was occupied with yolk (Fig. 3B). The digestive tract was a simple straight tube observed along the dorsal wall of the yolk. At 1 DAH (3.69 ± 0.16 mm TL), the lumen of the gut was slightly enlarged, and the digestive system of the chub mackerel was opened to the anus, although the mouth had not yet opened. The intestines and rectum were differentiated, and the mouth opened completely. The rudimentary swim bladder began to differentiate from the dorsal wall of the anterior gut at 2 DAH (3.91 ± 0.17 mm TL).
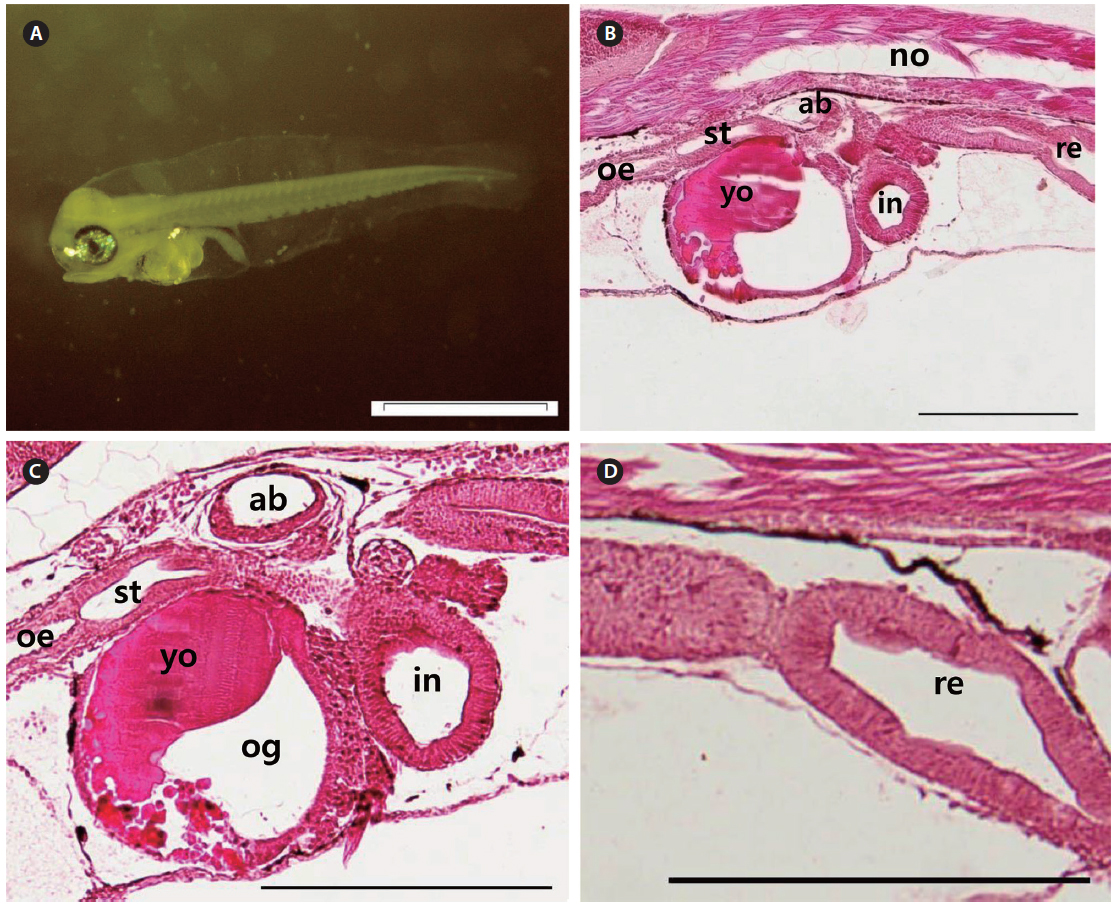
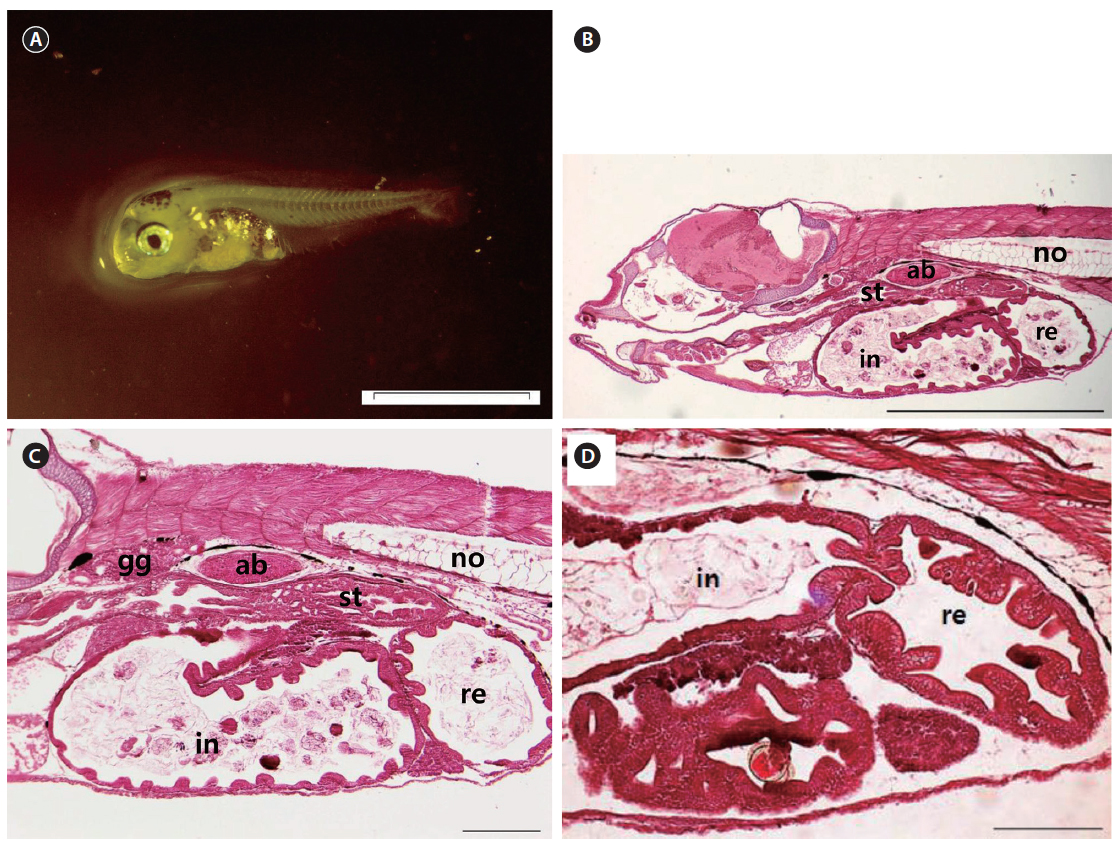
The mouth and anus were completely open, and the eyes fully pigmented at 3 DAH (3.91 ± 0.20 mm TL). The primordial fin fold, which had developed by 3 DAH, formed the external structure that was able to find and consume food. The digestive tract had expanded, with a rudimentary stomach apparent between the esophagus and the intestines at 3 DAH (Fig. 4A). The primitive structure of the digestive system was divided into an oropharyngeal cavity, esophagus, rudimentary stomach, swim bladder, intestines, and rectum. The gastric gland and blind sac were not observed in the stomach mucosa. Rotifers first appeared in the digestive tract at 3 DAH, and the basic structure of the digestive system, which enabled the larvae to ingest and digest exogenous nutrients, was established (Fig. 4B). The yolk was mostly absorbed, and the intestinal epithelium had thickened (Fig. 4C). The rectum epithelium was composed of columnar absorptive cells, with well-developed, striated borders (Fig. 4D). By 5 DAH (4.11 ± 0.23 mm TL) the intestines had become increasingly inflated, and the epithelium was composed of vacuoles. The rectum epithelium from which the mucosal fold does not develop, became thick, and acidophil granules were observed in great numbers. The rectum was completely separated from the intestines by a well-developed rectal valve. The first appearance of the gastric gland was noted at 5 DAH. By 7 DAH (4.67 ± 0.29 mm TL) the intestines had enlarged, and the mucosal fold of the rectum had started to developed. The mucosal folds of the stomach developed, the dorso-posterior section had enlarged to form the blind sac, and the pyloric caeca was differentiated. The mucosal folds of the intestines and rectum developed, and the intestines were divided into the anterior and posterior intestines by 9 DAH (5.24 ± 0.37 mm TL). The esophagus was differentiated at 11 DAH (5.53 ± 0.37 mm TL), with the stratified squamous epithelium in the front and simple squamous epithelium in the back. The number of gastric glands increased, and the stomach elongated posteriorly to form the blind sac at 11 DAH.
The head became larger, and the body depth increased. The notochord tip began to turn upward with the appearance of urophysial bones at 13 DAH (5.91 ± 0.45 mm TL) (Fig. 5A). Development of the digestive system as a whole took place at this time. The mucosal folds of the stomach in particular were developed (Fig. 5B). The mucosal epithelium became thicker and noticeably developed (Fig. 5C, D).
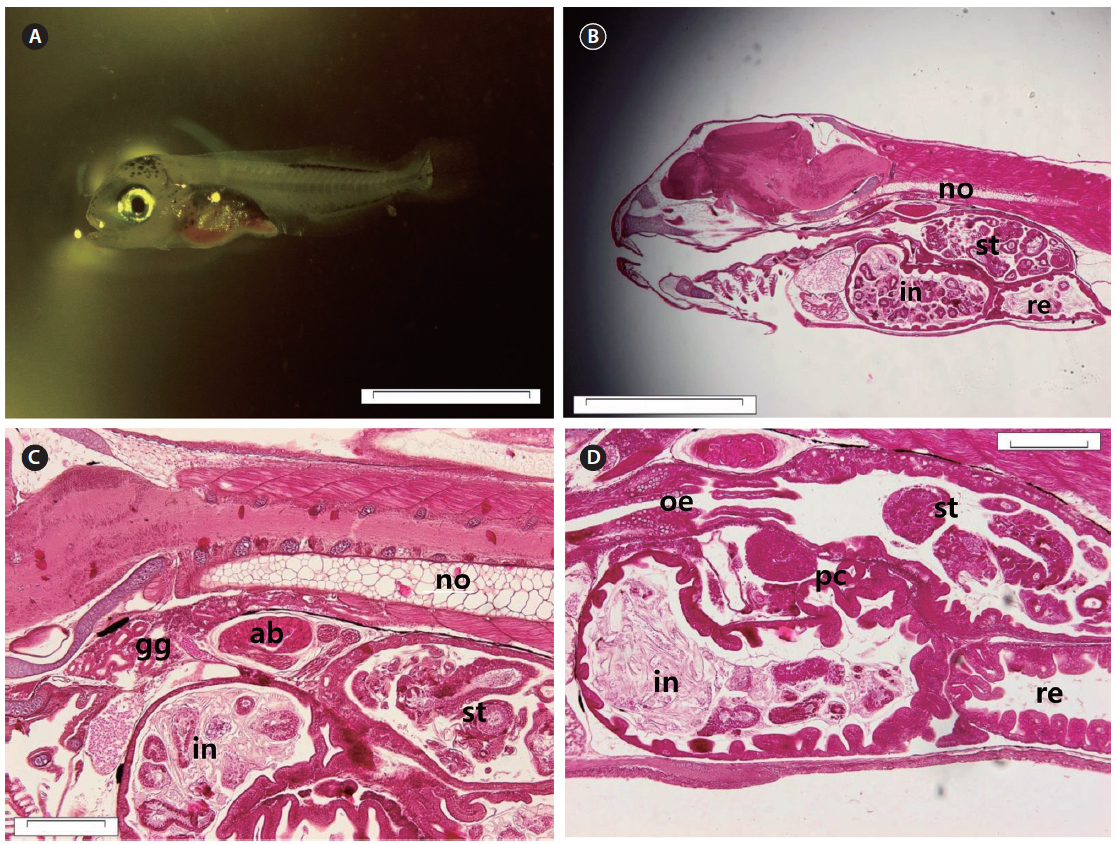
At 17 DAH (7.09 ± 0.61 mm TL), the notochord tip upturn was complete, along with a nearly complete caudal fin (Fig. 6A). The stomach had separated into the cardiac and pyloric portions and the blind sac. The blind sac extended posteroventrally (Fig. 6B). The number of gastric glands had increased (Fig. 6C). The number of jaw and pharyngeal teeth, gastric glands, and pyloric caeca had also increased. The pyloric caeca were well formed at the anterior-most part of the intestines. There were few mucous cells on the oral cavity epithelium (Fig. 6D).
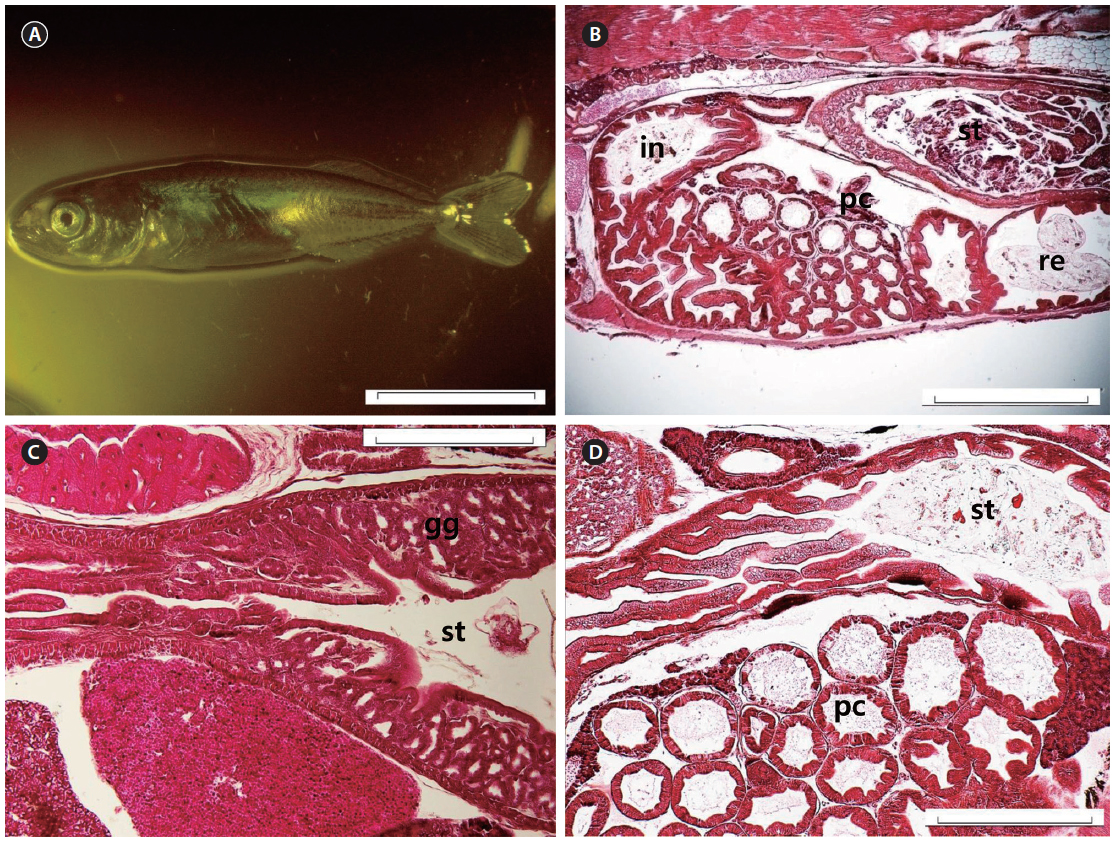
At 27 DAH, all the fins were fundamentally established (Fig. 7A), and the histological characteristics of the digestive organ were similar to those of adult chub mackerel (Fig. 7B). The gastric glands had increased in number (Fig. 7C) and were distributed throughout the stomach, with the exception of the pyloric portion (Fig. 7D). The gastric lumen contained a large volume of food, resulting in the expansion of the blind sac toward the posterior edge of the abdominal cavity. Intestinal epithelial cells were stained dark with hematoxylin in all the fish examined.
The development of the digestive system in
Pinocytosis has been suggested as an alternative pathway for the digestion of proteins in teleost larvae, as their enzymatic digestive system is poorly developed (Watanabe, 1982). This study showed that vacuoles appeared evenly on the intestinal epithelium of chub mackerel larvae, and acidophil granules were observed in the rectal epithelium at 3 DAH. Iwai and Tanaka (1968) and Tanaka (1973) reported that the vacuoles and acidophil granules were indicative of larval digestion and absorption mechanisms that existed before the development of the functionality of the early divided stomach and gastric gland of larval fish. In most marine fishes, the development of a digestive system consisting of a functional stomach with well-developed gastric glands and pyloric caeca occurs in association with the transformation to the juvenile stage. Accordingly, the typical development pattern of marine fishes involves a mechanism for digestion, and absorption changes from pinocytosis and intracellular digestion to extracellular digestion and membrane transport with the development of the gastric gland (Govoni et al., 1986).
The first appearance of the gastric gland was reported at 21 DAH in red sea bream, 15 DAH in rainbow trout (
Although five scombrid larvae, including the chub mackerel, showed species-specific timing in the appearance of the gastric glands and pyloric caeca, their digestive system attained the juvenile-type structure long before metamorphosis. On the other hand, a juvenile-type digestive system formed during the juvenile period, at 35 DAH, in black sea bream and false kelpfish (
Scombrids can grow very rapidly during their early life stages, and this may be related to the precocious development of the digestive system in this group of fishes (Tanaka et al., 1996). In chub mackerel, the total length and body weight increased gradually from 17 DAH; thereafter, there was a rapid increase from 25 DAH. As in other scombrids, chub mackerel larvae also undergo marked, rapid growth during the early life stages, which is supported by their precocious digestive system. In addition, Shoji et al. (2001) reported that the absolute growth of wild chub mackerel increased from 0.298 mm/day (post-first-feeding stage) to 0.712 mm/day (later larval and early juvenile stages), suggesting that the increase in growth rate of the chub mackerel may result from the shift in feeding to piscivory.
Tanaka et al. (1996) suggested that the precocious development of the digestive system with differentiation of the functional stomach in scombrid larvae also supported the appearance of piscivory. The increase in pepsin-like enzyme activity measured from the whole body extract is likely to be concomitant with gastric gland differentiation in the scombrid larvae, as in many other species (Govoni et al., 1986). This physiological advancement, as well as the formation of a blind sac that enables the larvae to store larger prey items, would support the appearance of piscivory in scombrid larvae, including chub mackerel larvae.
The peak occurrence of wild Spanish mackerel larvae is synchronized with the peak abundance of their prey larvae, suggesting the ecological advantage of early piscivory with the precocious development of the digestive system (Shoji et al., 1999). In contrast, the bluefin and yellowfin tuna larvae attained juvenile-type digestive systems during the flexion phase and underwent a larval period of planktivory with a larval-type digestive system, suggesting that they may have adapted to their offshore feeding habitat where the density of prey fish larvae may be low (Tanaka et al., 1996). Kohno et al. (1984) reported that swimming and feeding abilities develop rapidly after the development of the jaw and pharyngeal teeth, as well as the caudal fin formation in chub mackerel larvae of 5–7 mm SL. In addition, 25% of wild chub mackerel larvae measuring 5–6 mm SL contained fish in their stomachs; this increased to 80% at 9–10 mm SL (Shoji et al., 2001). As in the Spanish mackerel, the early morphological developments and precocious digestive system in chub mackerel larvae were likely associated with the early onset of piscivory. Stomach contents analysis of wild chub mackerel larvae of 4–9 mm TL showed that the main prey were the larvae of the sea squirt, crustaceans,