Mechanisms that determine the population densities of tree species in forest communities are central to investigations of forest ecology, and it is widely accepted that the Janzen–Connell model (Janzen 1970, Connell 1971) contributes to the maintenance of species diversity in plant communities (Gilbert 2002, Howe and Miriti 2004). It is the effect that seedling density is maximized at a certain distance from the tree, because dispersed seed density decreases as the distance from the mother tree increases, and the proportion of seeds escaping from specific mortality due to disease or predation increases as the distance from the tree increases. This mechanism has been investigated in numerous studies and although refuted in some (Burkey 1994, Hyatt et al. 2003), has been observed for many species in temperate and tropical forests (Clark and Clark 1984, Lambers et al. 2002, Petermann et al. 2008, Mangan et al. 2010, Shibata et al. 2010).
Although the Janzen–Connell model describes seed and seedling stages, species dominance during pre-seed dispersal stages is subject to additional mechanisms, including the contradictory density dependence of pollination and seed/embryo predation. Previous studies have shown that pollination efficiency is positively correlated with local conspecific density of reproductive individuals. Moreover, multiple insect-pollinated plants are subjected to a decreased attraction of pollinators following fragmentation or isolation of the population (Silander 1978, House 1993, Ghazoul et al. 1998, García and Chacoff 2007, Dauber et al. 2010). Pollen limitations were also reported for wind-pollinated plants (Knapp et al. 2001). However, high seed and embryo predation has been associated with high local conspecific density in a number of studies (Steffan-Dewenter et al. 2001, García and Chacoff 2007, De Crop et al. 2012).
A few studies have examined the dependence of local density on both pollination and seed/embryo predation (Silander 1978, Steffan-Dewenter et al. 2001, García and Chacoff 2007), and although Silander (1978) found a positive correlation between conspecific density and seed sets, no significant correlations were found between predation rates and density. Steffan-Dewenter et al. (2001) also reported a positive correlation between the number of flower-visiting bees and the population density of a plant, and reported a negative correlation between the proportions of flower heads that escaped predation and plant density. García and Chacoff (2007) similarly showed a positive correlation between seed set and tree cover density, but the proportion of seeds that escaped predation (by mice) was also positively correlated with cover density. Hence, predator satiation (Janzen 1971, Silvertown 1980) may influence the correlation between the proportion of seeds that escaped predation and conspecific density. Taken together, these studies suggest an instability in the relationships between predation and local plant density.
In the present study, we assessed the effects of conspecific density on the reproductive success of
This study was performed in the broad-leaved deciduous secondary forests of Inagi City, Tokyo, Japan. The study area was about 3.8 km2 and was located at 35°38′ N, 139°30′E. The mean annual temperatures of the nearest automated meteorological data acquisition system (AMeDAS) station, which was about 4 km away from the study site and had a similar elevation, was 15.0°C, and the monthly maximum and minimum temperatures of 26.5°C and 4.2°C occurred in August and January, respectively. The mean annual precipitation is 1,529.7 mm (Japan Meteorological Agency 1981-2010). The original vegetation before human disturbance was likely broadleaf evergreen forest and was dominated by trees belonging to the subgenus
>
Selection of target individuals and survey of all reproductive individuals
The 27 target individuals of
>
Collection and classification of seeds
Mature seeds of
We analyzed the relationship between local conspecific abundance and seed set (we assumed it nearly equal to pollination rate,
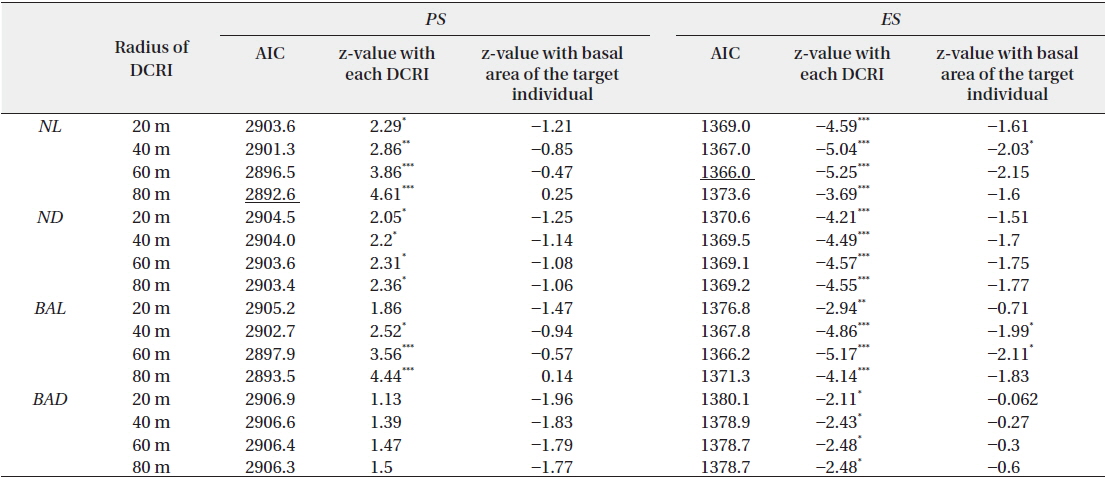
Four indices of local abundance of conspecific reproductive individuals were calculated from the population densities, distances from the target individuals, and basal areas. These included the following: 1) the sum of reproductive individuals within a particular distance from the target individual (
Subsequently, three indices for pollination and predation were applied as follows: 1)
The effects of local abundances of reproductive individuals (
Almost all individuals over 9-cm GBH reproduced during the year of observation, and Sound seeds comprised 17% (range, 1–54%) of all sampled seeds. In comparison with a previous study (Shibata et al. 2010), the year 2013 was one of the best out of recent seed years. The mean and range proportions of Predated, Empty, Immature, Broken, and Decayed seeds were 31% (0–63%), 27% (4–94%), 14% (0–43%), 7% (0–33%), and 4% (0–11%), respectively
The variables
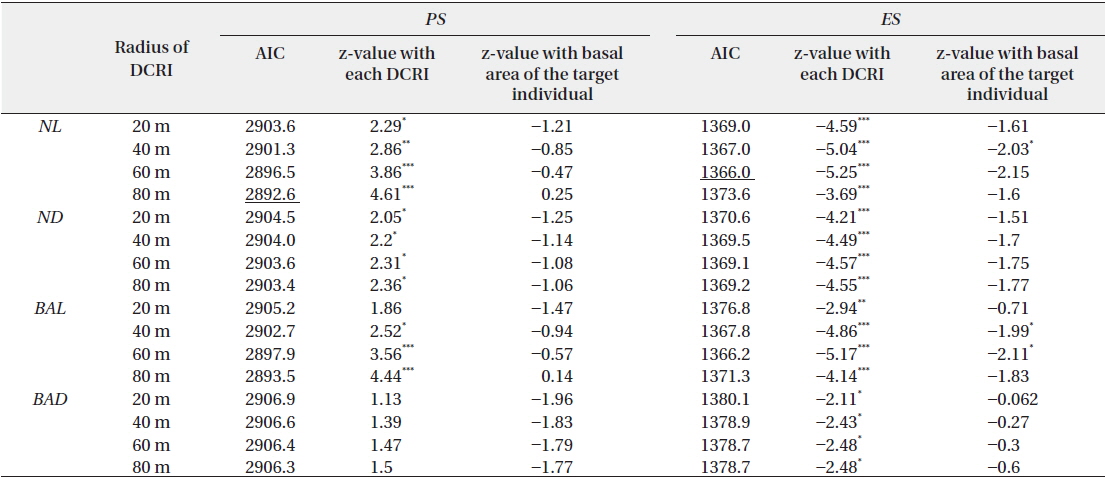
Effects of four indices of local densities of conspecific reproductive individuals (DCRI) and corresponding basal areas of target individuals on rates of pollination (PS) and escape from predation (ES)
Thus, large conspecific abundance corresponded with increased Predated seed, whereas small local conspecific abundance was associated with increased Empty seeds. Consequently,
>
The effect of local conspecific abundance on pollination efficiency
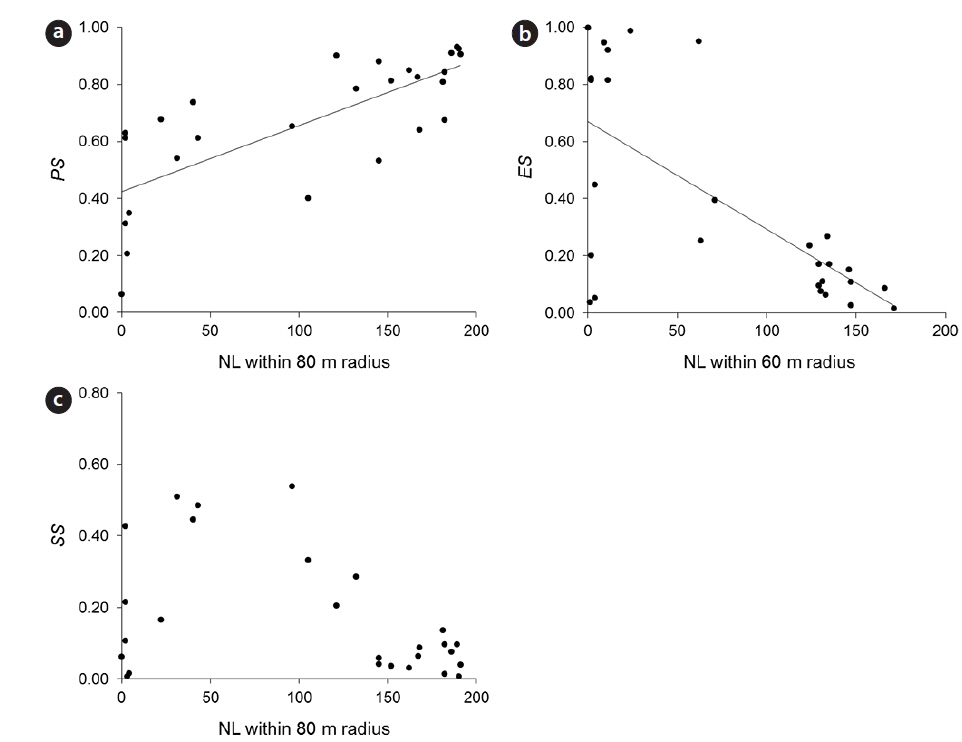
Empty seeds comprised of 27% of the total number of seeds, suggesting that fertilization greatly influences seed production. Moreover, the observed positive correlation between local conspecific abundance and seed sets suggests pollen limitation (Fig. 1a). From the present data, the distance with the strongest correlation efficient was estimated as 80 m. Among indices of local conspecific abundance, the number of reproductive individuals had the greatest effect on pollination efficiency, and that of basal areas of trees around the target individual and negatively weighted distances were less effective. The basal areas of individuals can be used to estimate the total amount of pollen produced by individual trees, and negative associations with the square of this distance may reflect distance effects. Although all of these indices can be used to make better estimations of seed set, the causes of differences between these indices remain unclear.
Pollen limitation during reproduction has been reported in multiple cases, and in agreement with Knapp et al. (2001), the data showed pollen limitation at 60–80 m in the wind-pollinated species
>
The effect of local conspecific abundance on predation rates
Predated seeds comprised of 31% of all seeds, suggesting that predation is an important factor for reproductive success. The proportion of seeds that escaped from predation was negatively correlated with the indices of local conspecific density (Fig. 1b), suggesting that population densities of
Annual fluctuations of seed production might have affected the present results, because during a good year of seed production, the proportion of seeds that escape predation tends to be high. This reflects the effects of predator satiation (Janzen 1971, Silvertown 1980) as confirmed for
The proportions of seeds that escaped predation were most strongly affected by the density of reproductive individuals within a 60-m radius. A previous study demonstrated that effective spatial scales of an insect-pollinated herb
>
Optimal conspecific densities for the production of Sound seeds
The best production of Sound
Taken together with previous studies, the present data indicate that although the maximum reproductive success in areas of moderate conspecific density occurs in only some cases, the associated mechanisms may be related to the control of population densities of plant communities.