There has been increasing interest in the use of microalgae as sources of renewable energy and other economically important products (Sushchik et al. 2003, Chisti 2007, Mata et al. 2010, Abou-Shanab et al. 2011).
Numerous studies have attempted to optimize the conditions for growth and lipid production in
Previous experiments indicated significant physiological differences among isolates of microalgae of the same species, possibly due to adaptations to local environments (Gallagher 1986, Lee and Kim 2007, Kim et al. 2009). Gallagher (1982) and Soudek and Robinson (1983) reported genetic, biochemical, and physiological differences of algae isolated during different seasons and from different geographical regions. The growth characteristics of a
The present research examined isolates of
>
Isolation and culture conditions
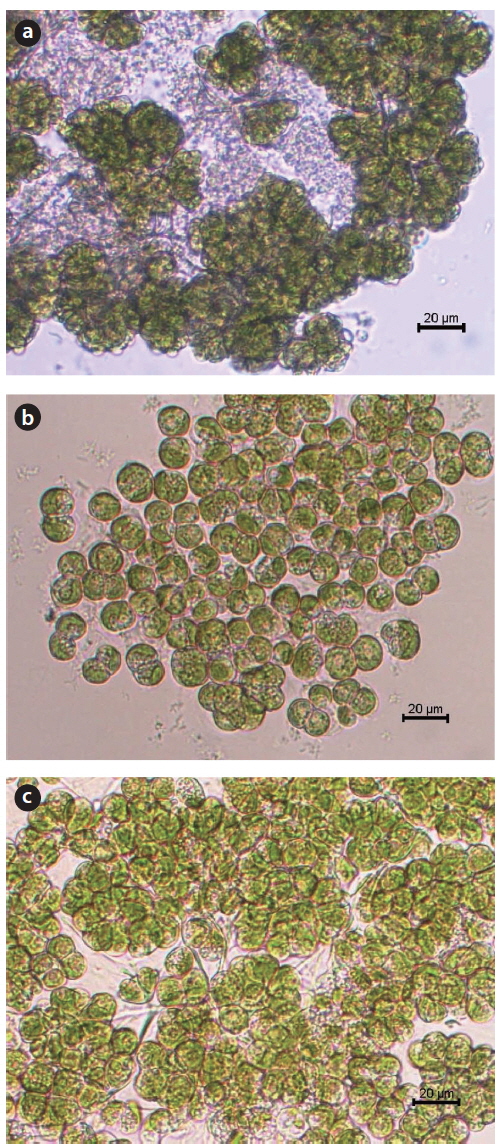
The Korean strains of

Geographic location, collection date, and collection site altitude of the 3 Korean strains of Botryococcus braunii examined in the present study
Uni-algal stock cultures were maintained at 24℃ under about 80 μmol m-2 s-1 of cool white fluorescent light on a 16 h light:8 h dark cycle in DY-III medium (Lehman 1976) with 100 mL min-1 aeration. DY-III medium contained the following components (g L-1): 0.02, CaCl2·2H2O; 0.074, MgSO4·7H2O; 0.01, Na2 glycerophosphate·5H20; 0.02, NaNO3; 0.015, NaSiO3·9H2O; 0.01, NH4NO3; 0.01, KCl; 0.0002, Thiamine; 0.0000005, Biotin; 0.0000005, Vitamin B12; and Trace metals solution. For growth medium experiments, the three strains were grown in DY-III, BG-11 (Stanier et al. 1971), and Chu-13 media (Largeau et al. 1980) at 24℃ under about 80 μmol m-2 s-1 of cool white fluorescent light on a 16 h light:8 h dark cycle with 100 mL min-1 aeration. BG-11 medium consisted of (g L-1): 1.5, NaNO3; 0.04, K2HPO4; 0.075, MgSO4·7H2O; 0.036, CaCl2·2H2O; 0.006, Citric acid; 0.0006, Ammonium ferric citrate; 0.001, EDTA-Na2; 0.02, Na2CO3; and Trace metals solution and Chu-13 medium contained the following components (g L-1): 0.4, KNO3; 0.08, K2HPO4; 0.2, MgSO4·7H2O; 0.107, CaCl2·2H2O; 0.1, Citric acid; 0.02, Ferric citrate; and Trace metals solution. For temperature experiments, the three strains were cultured in BG-11 medium at 15℃, 20℃, 25℃, and 30℃ under about 80 μmol m-2 s-1 of cool white fluorescent light on a 16 h light:8 h dark cycle with 100 mL min-1 aeration. All experiments were conducted in triplicate in 500-mL glass bottles after adaptation for two weeks in the selected growth medium and temperature.
>
Measurement of biomass and growth rate
For measurement of dry cell weight (DCW), 0.5 mL of each sample was filtered onto a weighed glass microfiber filter (25 mm GF/C; Whatman, Buckinghamshire, UK) every three days. These samples were washed with distilled water, dried at 70℃ for 24 h, and weighed with a XM 1000P microbalance (Sartorius, Goettingen, Germany). The growth rate (g day-1) is expressed as μ = ln(N2/N1)/(t2 - t1), where N2 and N1 are the DCW during the period of exponential growth at times t2 and t1, respectively (Levasseur et al. 1993).
>
Extraction and analysis of total lipids
The total lipids produced at each temperature were extracted with a modification of the method described by Bligh and Dyer (1959), using a chloroform:methanol ratio of 1:2. The chloroform layer was separated and evaporated in the clean bench, and the remaining extract was weighed with a XM 1000P microbalance (Sartorius).
The lipid components were measured by gas chromatography (GC-2010; Shimadzu, Kyoto, Japan). First, 50 mg of each sample was saponified by addition of 1 mL of the saturated KOH-CH3OH solution at 75℃ for 10 min, and then submitted to methanolysis with 5% HCl in methanol at 75℃for 10 min. Then, the fatty acid layer was separated by adding 2 mL of distilled water. The peaks of the nine lipid components were identified by comparison with standards (Xu et al. 2001).
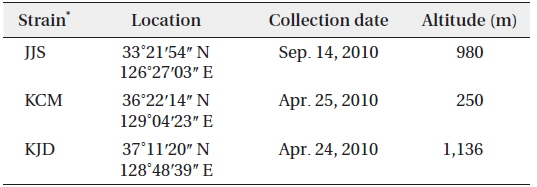
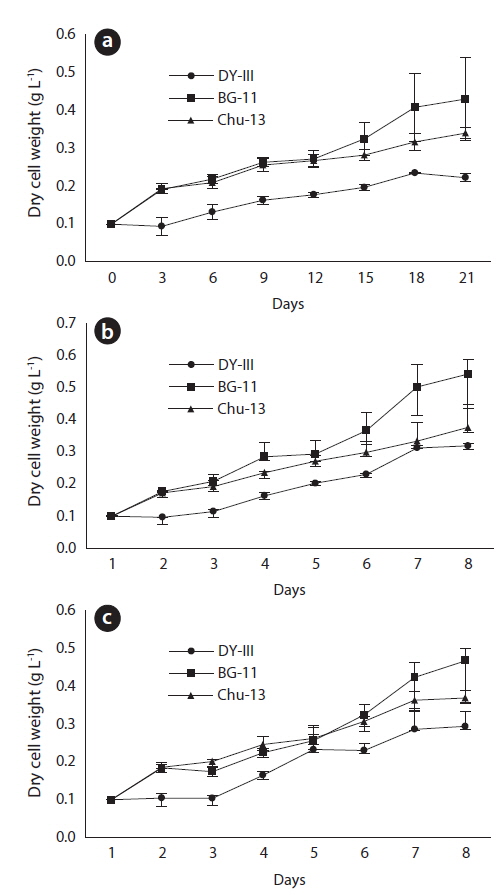
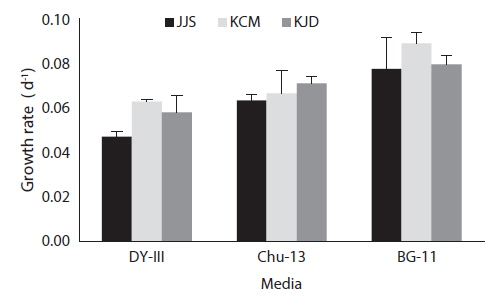
After 21 days of growth, in the JJS strain, the DCW and the growth rate were greatest in BG-11 medium (0.430 g L-1 and 0.078 g day-1) and lowest in DY-III medium (0.222 g L-1 and 0.047 g day-1) (Figs. 2a and 3). In the KCM strain, the DCW and the growth rate were greatest in BG-11 medium (0.543 g L-1 and 0.090 g day-1) and lowest in DY-III medium (0.319 g L-1 and 0.063 g day-1) (Figs. 2b and 3). In the KJD strain, the DCW and the growth rate were greatest in BG-11 medium (0.457 g L-1 and 0.080 g day-1) and lowest in DY-III medium (0.294 g L-1 and 0.058 g day-1) (Figs. 2c and 3).
>
Growth at different temperatures
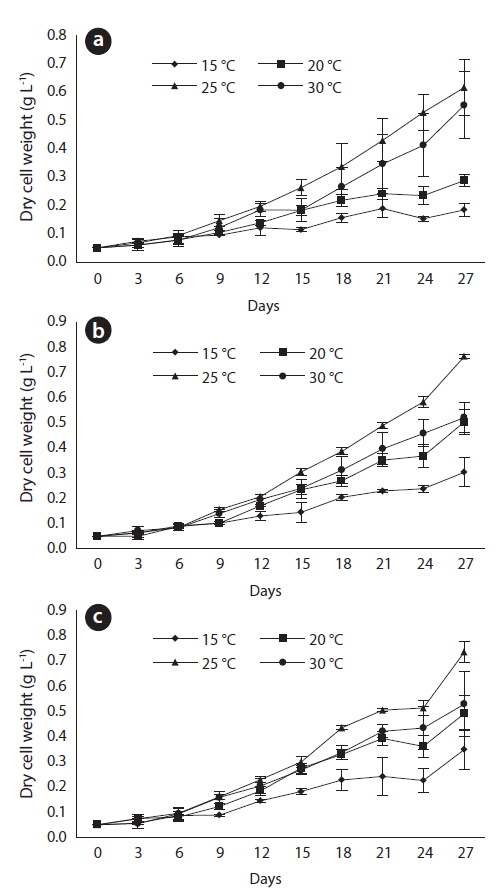
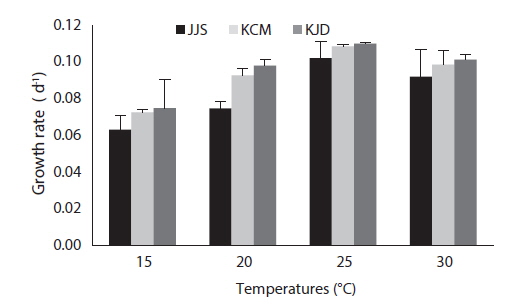
After 27 days of growth, in the JJS strain, the DCW and the growth rate were greatest at 25℃ (0.616 g L-1 and 0.102 g day-1) and lowest at 15℃ (0.184 g L-1 and 0.063 g day-1) (Fig. 4a and 5). In the KCM strain, the DCW and the growth rate were greatest at 25℃ (0.763 g L-1 and 0.109 g day-1) and lowest at 15℃ (0.304 g L-1 and 0.073 g day-1) (Figs. 4b and 5). In the KJD strain, the DCW and the growth rate were greatest at 25℃ (0.735 g L-1 and 0.110 g day-1) and lowest at 15℃ (0.349 g L-1 and 0.075 g day-1) (Figs. 4c and 5).
>
Total lipid content at different growth temperatures
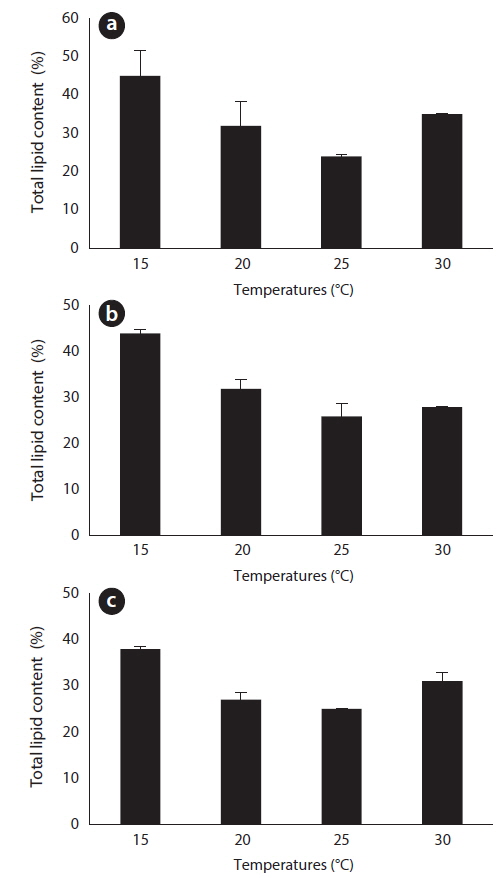
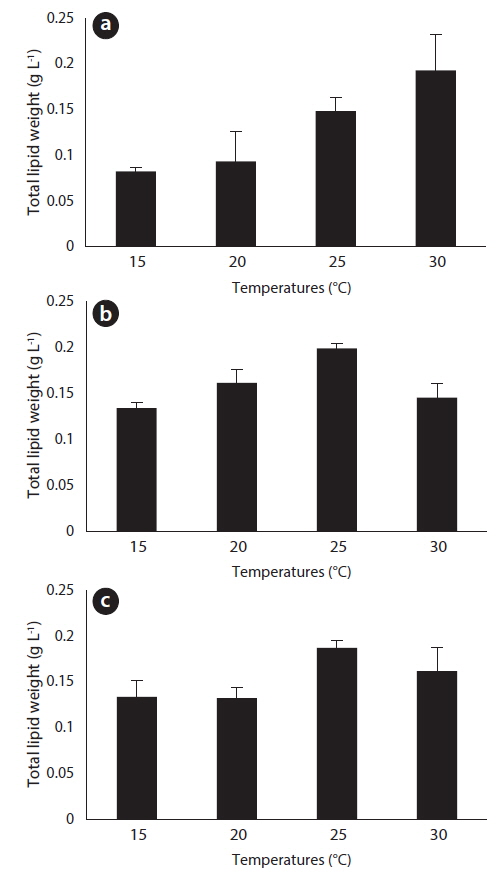
After 27 days of growth, in the JJS strain, it had the greatest percent dry weight of lipids at 15℃ (45%) and the lowest at 25℃ (24%) (Fig. 6a); the total lipid yield was greatest at 30℃ (0.193 g L-1) and lowest at 15℃ (0.083 g L-1) (Fig. 7a). In the KCM strain, it had the greatest percent dry weight of lipids at 15℃ (44%) and the lowest at 25℃ (26%) (Fig. 6b); the total lipid yield was greatest at 25℃ (0.199 g L-1) and lowest at 15℃ (0.134 g L-1) (Fig. 7b). In the KJD strain, it also had the greatest percent dry weight of lipids at 15℃ (38%) and the lowest at 25℃ (25%) (Fig. 6c); the total lipid yields were similar at temperatures below 20℃ (0.132-0.134 g L-1) and were maximal at 25° C (0.187 g L-1) (Fig. 7c).
>
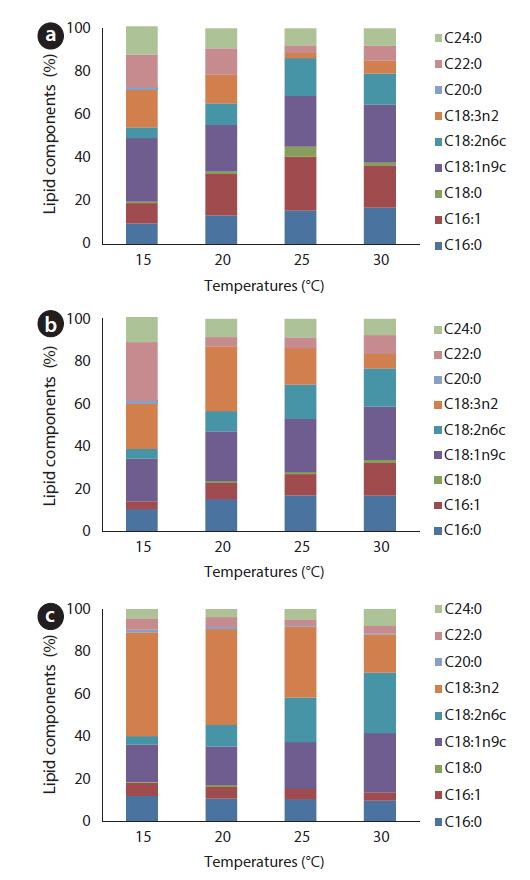
Lipid profiles at different growth temperatures
For all tested temperatures except 25℃, the amount of oleic acid (C18:1) was greatest in the JJS strain; at 25℃, the amount of palmitoleic acid (C16:1) was greatest, and oleic acid was second greatest (Fig. 8a). In the KCM strain, the amount of oleic acid was greatest at relatively high temperatures (25-30℃); linolenic acid (C18:3) was greatest at 15℃, behenic acid (C22:0) was greatest at 20℃, and oleic acid was second greatest at these two temperatures (Fig. 8b). The KJD strain produced the greatest amount of oleic acid at 30℃; at temperatures below 25℃, the amount of linolenic acid was greatest, and the proportion of oleic acid was second greatest (Fig. 8c).
Previous studies used the Chu-13 medium (Sawayama et al. 1994, Dayananda et al. 2005, Ranga Rao et al 2007, Ashokkumar and Rengasamy 2012) or the BG-11 medium (Dayananda et al. 2007, Ge et al. 2011) for culturing of
Lupi et al. (1991) reported that
The effect of temperature on the lipid content and profile were also analyzed to determine the optimal culture conditions for biodiesel production. All strains had the greatest amount of lipids per g DCW at 15℃ and the lowest amount at 25℃. Kalacheva et al. (2002) reported that environmental conditions (e.g., temperature or light intensity) which favor a high growth rate in
The lipids extracted from microalgae consist of various fatty acids (Zhila et al. 2005a, 2005b). Oleic acid (C18:1) is easily converted into biodiesel due to its low melting point (-20℃) and strong stability (Knothe 2008). The present study examined the 9 lipid components, including oleic acid, palmitoleic acid (C16:1), and linolenic acid (C18:3), that were produced by three Korean strains of
Taken together, the results of this study indicate that these three Korean strains of